
ORGANIC CHEMISTRY
6th Edition
ISBN: 9781266633973
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 56P
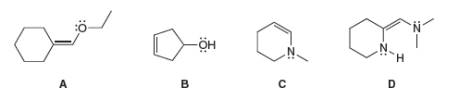
Consider compounds A-D, which contain both a heteroatom and a double bond. (a) For which compounds are no additional Lewis structures possible? (b) When two or more Lewis structures can be drawn, draw all additional resonance structures.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A stable triatomic molecule can be formed that contains one atom each of nitrogen, sulfur, and fluorine. Three bonding structures are possible, depending on which is the central atom: NSF, SNF, and SFN.
(a) Write a Lewis diagram for each of these molecules, indicating the formal charge on each atom.
(b) Often, the structure with the least separation of formal charge is the most stable. Is this statement consistent with the observed structure for this molecule— namely, NSF, which has a central sulfur atom?
(c) Does consideration of the electronegativities of N, S, and F from Figure 3.18 help rationalize this observed structure? Explain. 100. The gas
Consider compounds A–D, which contain both a heteroatom and a double bond.
(a) For which compounds are no additional Lewis structures possible?
(b) When two or more Lewis structures can be drawn, draw all additional resonance structures.
A stable triatomic molecule can be formed that containsone atom each of nitrogen, sulfur, and fluorine. Threebonding structures are possible, depending on which is thecentral atom: NSF, SNF, and SFN.(a) Write a Lewis diagram for each of these molecules,indicating the formal charge on each atom.(b) Often, the structure with the least separation of formal charge is the most stable. Is this statement consistent with the observed structure for this molecule—namely, NSF, which has a central sulfur atom?(c) Does consideration of the electronegativities of N, S,and F from Figure 3.18 help rationalize this observedstructure? Explain.
Chapter 1 Solutions
ORGANIC CHEMISTRY
Ch. 1.1 - While the most common isotope of nitrogen has a...Ch. 1.2 - Label each bond in the following compounds as...Ch. 1.3 - Draw a valid Lewis structure for each species. a....Ch. 1.3 - Prob. 9PCh. 1.4 - Draw Lewis structures for each molecular formula....Ch. 1.6 - Classify each pair of compounds as isomers or...Ch. 1.6 - Prob. 12PCh. 1.6 - Prob. 13PCh. 1.6 - Prob. 14PCh. 1.6 - Prob. 16P
Ch. 1.6 - Prob. 17PCh. 1.7 - Prob. 18PCh. 1.7 - Prob. 19PCh. 1.7 - Using the principles of VSEPR theory, you can...Ch. 1.8 - Convert each condensed formula to a Lewis...Ch. 1.8 - Prob. 22PCh. 1.8 - Prob. 23PCh. 1.8 - Convert each skeletal structure to a complete...Ch. 1 - Citric acid is responsible for the tartness of...Ch. 1 - Zingerone gives ginger its pungent taste. a.What...Ch. 1 - Assign formal charges to each and atom in the...Ch. 1 - Prob. 44PCh. 1 - Prob. 46PCh. 1 - Draw all possible isomers for each molecular...Ch. 1 - 1.45 Draw Lewis structures for the nine isomers...Ch. 1 - Prob. 52PCh. 1 - Prob. 53PCh. 1 - Prob. 54PCh. 1 - Consider compounds A-D, which contain both a...Ch. 1 - Draw in all the carbon and hydrogen atoms in each...Ch. 1 - 1.61 Convert each molecule into a skeletal...Ch. 1 - Prob. 65PCh. 1 - Predict the hybridization and geometry around each...Ch. 1 - Prob. 68PCh. 1 - Ketene, , is an unusual organic molecule that has...Ch. 1 - Rank the following bonds in order of increasing...Ch. 1 - Two useful organic compounds that contain Cl atoms...Ch. 1 - Use the symbols + and to indicate the polarity of...Ch. 1 - Prob. 74PCh. 1 - Anacin is an over-the-counter pain reliever that...Ch. 1 - 1.77 Stalevo is the trade name for a medication...Ch. 1 - 1.78 and are two highly reactive carbon...Ch. 1 - 1.79 The N atom in (acetamide) is hybridized,...Ch. 1 - Prob. 83PCh. 1 - Prob. 84PCh. 1 - Prob. 85PCh. 1 - Prob. 86PCh. 1 - Prob. 87PCh. 1 - Prob. 88P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Draw a Lewis structure for each covalent molecule. a. HBr b. CH3F c. H2O2 d. N2H4 e. C2H6 f. CH2Cl2
Principles of General, Organic, Biological Chemistry
Real walls are never totally adiabatic. Use your experience to order the following walls in increasing order wi...
Thermodynamics, Statistical Thermodynamics, & Kinetics
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry
Practice Problem 1.22 Which of the following alkenes can exist as cis-trans isomers? Write their structures. Bu...
Organic Chemistry
For each of the following 2-dimensional shapes, determine the highest order rotation axis of symmetry.
Inorganic Chemistry
Characterize each of the following structures as aromatic, nonaromatic, or antiaromatic:
Answer: _____
Organic Chemistry As a Second Language: Second Semester Topics
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3) The molecule diphosphorus tetraoxide (P,O,) has two central atoms and four different resonance structures that do not violate the octet rule. Draw two of these resonance structures below. 4) The compound acetone is a common solvent. It has a chemical formula of CH,COCH, Acetone has three central atoms. (a) Draw the Lewis Dot structure for acetone. (b) Give the Ideal Bond Angle for all three central atoms. 5) Four covalent molecules are drawn below. :o: H. H-CH H H (1) (2) (3) (4) a) Define each of these molecules as polar or non-polar. (1) (2) (3) b) Describe the type of intermolecular force that each molecule would use: (1) (2) (3) (4)arrow_forwardFinish the following questions. ((a) Draw all of the possible Lewis structures (including reasonance structures) of the following compounds.(b) Label the formal charge for each atom.(c) Determine which resonance structure(s) is(are) the better/best and briefly explain. ClO2F2+arrow_forwardChemistry (a) Write three more resonance structures for each of compounds 1 and 2. (b) In each of compounds 1 and 2, determine which resonance structure contributes the most and explain your answer. (c) Are the 3/4 structures resonance structures or different compounds? Same question for 5/6 structures. Explain your answers.arrow_forward
- Some chemists believe that satisfaction of the octet rule should be the top criterion for choosing the dominant Lewis structure of a molecule or ion. Other chemists believe that achieving the best formal charges should be the top criterion. Consider the dihydrogen phosphate ion, HaPO, , in which the H atoms are bonded to O atoms. (a) What is the predicted dominant Lewis structure if satisfying the octet rule is the top eriterion? (b) What is the predicted dominant Lewis structure if achieving the best formal charges is the top criterion?arrow_forwardDraw Lewis structures for each of the following compounds. In each case, specify the number of valence electrons surrounding the central atom. (Assign lone pairs and radical electrons where appropriate.) (Assume the central atom does not contain an expanded octet.) (a) bromine dioxide (BrO2) (b) beryllium bromide (BeBr2) (c) phosphorus pentafluoride (PF5)arrow_forwardThe partial Lewis structure that follows is for a hydrocarbonmolecule. In the full Lewis structure, each carbon atomsatisfies the octet rule, and there are no unshared electronpairs in the molecule. The carbon—carbon bondsare labeled 1, 2, and 3. (a) How many hydrogen atomsare in the molecule? (b) Rank the carbon–carbonbonds in order of increasing bond length. (c) Whichcarbon—carbon bond is the strongest one?arrow_forward
- The partial Lewis structure that follows is for a hydrocarbonmolecule. In the full Lewis structure, each carbon atomsatisfies the octet rule, and there are no unshared electronpairs in the molecule. The carbon—carbon bondsare labeled 1, 2, and 3. (a) How many hydrogen atomsare in the molecule? (b) Rank the carbon–carbonbonds in order of increasing bond length. (c) Whichcarbon—carbon bond is the strongest one? [Sections 8.3and 8.8]arrow_forwardThe molecular ion S3N, has the cyclic structure 'N All S-N bonds are equivalent. (a) Give six equivalent resonance hybrid Lewis diagrams for this molecular ion. (b) Compute the formal charges on all atoms in the molecular ion in each of the six Lewis diagrams. (c) Determine the charge on each atom in the polyatomic ion, assuming that the true distribution of electrons is the average of the six Lewis diagrams arrived at in parts (a) and (b). (d) An advanced calculation suggests that the actual charge resident on each N atom is –0.375 and on each S atom is +0.041. Show that this result is consis- tent with the overall +1 charge on the molecular ion. Z-Sarrow_forwardThe structure at the right is a skeleton of an anion having the overall formula C6H,NO¯. The hydrogen atoms are not shown. (a) Draw a complete Lewis structure in which the -1 formal charge is on N. Include all H atoms and C. valence electrons. (b) Do the same for a Lewis structure with the -1 formal charge on O. (c) Do the same for a Lewis structure with the -1 formal charge on the C atom that is bonded to three other C atoms.arrow_forward
- (a) Describe the molecule xenon trioxide, XeO3, using four possible Lewis structures, one each with zero, one, two, or three Xe—O double bonds. (b) Do any of these resonance structures satisfy the octet rule for every atom in the molecule? (c) Do any of the four Lewis structures have multiple resonance structures? If so, how many resonance structures do you find? (d) Which of the Lewis structures in (a) yields the most favorable formal charges for the molecule?arrow_forward(a) Draw a second resonance structure for A. (b) Why can't a second resonance structure be drawn for B?arrow_forwardThe hypochlorite ion, ClO-, is the active ingredient inbleach. The perchlorate ion, ClO4-, is a main componentof rocket propellants. Draw Lewis structures for both ions. (a) What is the formal charge of Cl in the hypochlorite ion?(b) What is the formal charge of Cl in the perchlorate ion, assumingthe Cl—O bonds are all single bonds? (c) What is theoxidation number of Cl in the hypochlorite ion? (d) Whatis the oxidation number of Cl in the perchlorate ion, assumingthe Cl—O bonds are all single bonds? (e) In a redox reaction,which ion would you expect to be more easily reduced?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY