
Concept explainers
Citric acid is responsible for the tartness of citrus fruits, especially lemons and limes.
a. What is the molecular formula of citric acid?
b. How many lone pairs are present?
c. Draw a skeletal structure.
d. How many
e. What orbitals are used to form each indicated bond
(a)
Interpretation: The molecular formula of citric acid is to be stated.
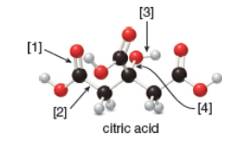
Concept introduction: In ball-and-stick model, each colored ball represents a specific atom and each stick represents a bond. In this model, each black ball represents
Answer to Problem 40P
The molecular formula of citric acid is
Explanation of Solution
The given ball-and-stick model of citric acid is,
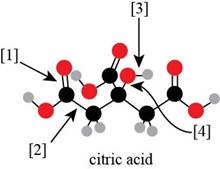
Figure 1
In ball-and-stick model, each colored ball represents a specific atom and each stick represents a bond. In this model, each black ball represents
In the above model,
• There are seven red balls. Thus, there are seven
• There are six black balls. Thus, there are six
• There are eight grey balls. Thus, there are eight
Hence, the molecular formula of citric acid is
The molecular formula of citric acid is
(b)
Interpretation: The number of lone pairs present in citric acid is to be stated.
Concept introduction: In a compound or molecule, the lone pairs represent number of unshared electrons on atom. An atom may or may not have unshared electrons. For example, carbon and hydrogen atoms have no lone pair but each oxygen atom has two lone pairs.
Answer to Problem 40P
There are total
Explanation of Solution
The molecular formula of citric acid is
There are total
(c)
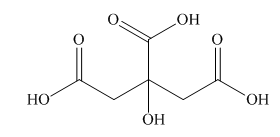
Interpretation: A skeletal structure of citric acid is to be drawn.
Concept introduction: A ball-and-stick model is converted into skeletal structure by replacing black ball with
Answer to Problem 40P
A skeletal structure of citric acid is,
Explanation of Solution
In ball-and-stick model each colored ball represents a specific atom and each stick represents a bond. A ball-and-stick model is converted into skeletal structure by replacing black ball with
A skeletal structure of citric acid is shown in Figure 2.

Figure 2
In ball-and-stick model each colored ball represents a specific atom and each stick represents a bond.
(d)
Interpretation: The number of
Concept introduction: According to the rule of hybridization, an atom that is surrounded with two groups is
Answer to Problem 40P
There are three
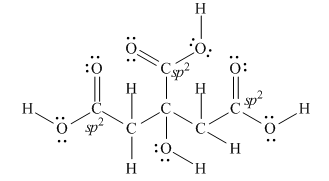
Explanation of Solution
The Lewis structure of citric acid is,
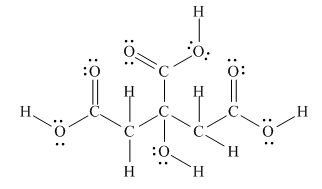
Figure 3
According to the rules of hybridization, an atom that is surrounded with two groups is
The

Figure 4
Thus, there are three
There are three
(e)
Interpretation: The orbitals that are used to form each indicated bond is to be stated.
Concept introduction: According to the rule of hybridization, an atom that is surrounded with two groups is
Answer to Problem 40P
Bond
Explanation of Solution
Bond [1] represents
Bond
Thus,
Bond
Thus,
Bond
The number of surrounded group around any atom predicts the hybridization of that atom, which is further helpful to predict the orbitals involve in bond formation.
Want to see more full solutions like this?
Chapter 1 Solutions
ORGANIC CHEMISTRY
Additional Science Textbook Solutions
Principles of Chemistry: A Molecular Approach (3rd Edition)
Fundamentals of Heat and Mass Transfer
General, Organic, & Biological Chemistry
Chemistry: The Central Science (13th Edition)
General, Organic, and Biological Chemistry (3rd Edition)
Chemistry & Chemical Reactivity
- Consider the following computer-generated model of caffeine: Complete a Lewis structure for caffeine in which all atoms have a formal charge of zero (as is typical with most organic compounds). How many C and N atoms are sp2 hybridized? How many C and N atoms are sp3 hybridized? sp hybridized? How many and bonds are there?arrow_forwardExplain what is wrong with each of the following statements. a. “A bond is a double bond. b. “A bond consists of four electrons, one in each of the four p orbitals involved in the bond. c. “A bond is twice as strong as a bond because it consists of two orbital overlaps insteadof just one.”arrow_forwardIn the hydrocarbon(a) What is the hybridization at each carbon atom in themolecule? (b) How many s bonds are there in the molecule?(c) How many p bonds? (d) Identify all the 120° bond anglesin the molecule. [Section 9.6]arrow_forward
- - What orbital(s) are the lone pairs of electrons on the O atom placed? - What orbital is the lone pair of electrons on the N atom placed? - What type of bond(s) are formed between Ca1 – C? - What type of bond(s) are formed between C = O? - What type of bond(s) are formed between C – N? - What type of bond(s) are formed between N – Ca2? - What type of bond(s) are formed between N – H? - What is the angle between Ca1 – C = O? - What is the angle between Ca1 – C – N? - What is the angle between O = C – N? - What is the angle between C – N – Ca2?arrow_forwardOne of the first drugs to be approved for use in treatment of acquired immune deficiency syndrome (AIDS) was azidothymidine (AZT). Complete the Lewis structure for AZT. a. How many carbon atoms are sp3 hybridized? b. How many carbon atoms are sp2 hybridized? c. Which atom is sp hybridized? d. How many σ bonds are in the molecule? e. How many π bonds are in the molecule? f. Wnat isthe N9N9N bond angle inthe azide (-N3) group? g. What is the H-Q-C bond angle in the side group attached to the five membered ring? h. What is the hybridization of the oxygen atom in the -CH2OH group?arrow_forwardFill in each blank sentence with numbers 0-10. An sp3-hybridized atom possess _____ s orbital(s), _______ p orbital(s), and _____ hybrid orbital(s) in its valence shell. Such an atom can form up to _____ sigma bonds and _____ pi bonds.arrow_forward
- One of the first drugs to be approved for use in treatment of acccquired immune deficiency syndrome (AIDS) was azidothymidine (AZT). Complete the Lewis structure for AZT a. How many carbon atoms are sp3 hybridised? b. How many carbon atoms are sp2 hybridised? c. Which atom is sp hybridised? d. How many σ bonds are in the molecule? e. How many π bonds are in the molecule? f. What is the N9N9N bond angle in the azide (--N3) group? g. What is the H--Q--C bond angle in the side group attached to the five-membered ring? h. What is the hybridization of the oxygen atom in the --CH2OH group?arrow_forwardDraw orbital diagrams (boxes with arrows in them) to represent the electron configurations—without hybridization—for all the atoms in SF2. Circle the electrons involved in bonding. Draw a three-dimensional sketch of the molecule and show orbital overlap. What bond angle do you expect from the unhybridized orbitals? How well does valence bond theory agree with theexperimentally measured bond angle of 98.2°?arrow_forwardWhat are the hybridizations of carbon atoms 1, 2, and 3 shown in the model below? H2C = CH - CH3 a. sp 2, sp 2, sp 3 b. sp, sp, sp 2 c. sp2, sp, sp 3 d. sp, sp 2, sp 2arrow_forward
- How many sp2 hybridized carbons are present?arrow_forwardHow many pi bonds? How many unhybridized p-orbitals? How many sp-orbitals? How many sp2-orbitals?arrow_forward1. How many sp^3 orbitals does your oxygen have? * 2. How many Pi bond/s connect/s your Oxygen to your Carbon? * 3. How many sigma bond/s connect/s your Oxygen to your Carbon? * 4. Which line representation would tell the viewer of which hydrogen is toward him/her (closest to the viewer)? *A. AB. BC. CD. None of the above 5. What hybrid orbital do all your central atoms have? *A. spB. sp^2C. sp^3D. spd^3 6. If we are to consider only your C-O-C, what geometry will they form? *A. tetrahedralB. trigonal pyramidalC. trigonal planarD. linearE. bent 7. What angle will be closest to the angle of number 1? *A. 180B. 109.5C. 120D. 90 8. What angle will be closest to the angle of number 2? *A. 180B. 109.5C. 120D. 90 9. How many hybrid orbitals that formed covalent bonds can you count? *1 pointA. 6B. 7C. 8D. 9E. 10F. 11 10. How many atoms were bonded covalently? *arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





