
Concept explainers
Consider
M aqueous solutions of each of the following. Which solution is more basic?
Sodium cyanide
or sodium fluoride
Sodium carbonate
or sodium acetate 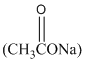
Sodium sulfate
or sodium methanethiolate
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Organic Chemistry - Standalone book
- Classify each of the following acids as monoprotic, diprotic, or triprotic. a. H2SO4 (sulfuric acid) b. HC2H3O2 (acetic acid) c. H2C5H6O4 (glutaric acid) d. HCN (cyanic acid)arrow_forwardCalculate the concentration of all solute species in each of the following solutions of acids or bases. Assume that the ionization of water can be neglected, and show that the change in the initial concentrations can be neglected, Ionization constants can be found in Appendix H and Appendix I. (a) 0.0092 M HCIO, a weak acid. (b) 0.0784 M C6H5NH2, a weak base. (c) 0.0810 M HCN, a weak acid. (d) 0.11 M (CH3)3N, a weak base. (e) 0.120 M Fe(H2O)62+ a weak acid, Ka=1.6107arrow_forwardWhich of the solutions listed below has the lowest pH? (a) 0.10 M HCl (b) 0.10 M NaOH (c) 2.5 105 M HNO3 (d) pure H2Oarrow_forward
- Consider the following six beakers. All have 100 mL of aqueous 0.1 M solutions of the following compounds: beaker A has HI beaker B has HNO2 beaker C has NaOH beaker D has Ba(OH)2 beaker E has NH4Cl beaker F has C2H5NH2 Answer the questions below, using LT (for is less than), GT (for is greater than), EQ (for is equal to), or MI (for more Information required). (a) The pH in beaker A the pH in beaker B. (b) The pH in beaker C the pH in beaker D. (c) The % ionization in beaker A the % ionization in beaker C. (d) The pH in beaker B the pH in beaker E. (e) The pH in beaker E the pH in beaker F. (f) The pH in beaker C the pH in beaker F.arrow_forwardIndicate whether solutions of each of the following substances contain ions, molecules, or both. a. acetic acid, a weak acid b. sucrose, a nonelectrolyte c. sodium sulfate, a soluble salt d. hydrofluoric acid, a weak electrolytearrow_forwardThe active ingredient formed by aspirin in the body is salicylic acid, C6H4OH(CO2H). The carboxyl group. (-CO2H) acts as a weak acid. The phenol group (an OH group bonded to an aromatic ring) also acts as an acid but a much weaker acid. List, in order of descending concentration, all of the ionic and molecular species present in a 0.001-M aqueous solution of C6H4OH(CO2H).arrow_forward
- Which of the following conditions indicate a basic solution? pOH = 11.21 pH = 9.42 (OH’] > IH+| |OH] > 1.0 X IO’7Marrow_forwardIn each of the following acid-base reactions, identify the Brnsted acid and base on the left and their conjugate partners on the right. (a) HCO2H(aq) + H2O() HCO2(aq) + H3O+(aq) (b) NH3(aq) + H2S(aq) NH4+(aq) + HS(aq) (c) HSO4(aq) + OH(aq) SO42(aq) + H2O+()arrow_forwardClassify each of the following salts as a strong acidstrong base salt, a strong acidweak base salt, a weak acidstrong base salt, or a weak acidweak base salt. a. K3PO4 b. NaNO3 c. KCl d. Na2C2O4arrow_forward
- For each of the following pairs of solutions, indicate whether the first listed solution has a higher or lower pH than the second listed solution. a. 1.0 M NaOH and 1.0 M HCl b. 1.0 M HNO3 and 0.10 M HNO3 c. 0.10 M HClO4 and 0.10 M HCN d. [H3O+] = 3.3 103 and [H3O+] = 9.3 103arrow_forwardIn each of the following acid-base reactions, identify the Brnsted acid and base on the left and their conjugate partners on the right. (a) C2H5N(aq) + CH3CO2H(aq) C5H5NH+(aq) + CH3CO2(aq) (b) N2H4(aq) + HSO4(aq) N2H5+(aq) + SO42(aq) (c) [Al(H2O)6]3+ (aq) + OH(aq) [Al(H2O)5OH]2+ (aq) + H2O+()arrow_forwardMagnesium hydroxide and magnesium citrate function as mild laxatives when they reach the small intestine. Why do magnesium hydroxide and magnesium citrate, two very different substances, have the same effect in your small intestine. (Hint: The contents of the small intestine are basic.)arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





