
Concept explainers
Interpretation:
The equation that shows the reaction of the given acid with water by considering the Bronsted-Lowry acid-base theory is to be written. All the electron pairs, formal chargers and curved arrows that represent the electron movement in the respective reaction are to be shown.
Concept introduction:
An acid is a chemical substance that readily donates protons and a base is a chemical substance that can easily accept a proton. During an acid-base reaction, the interaction between an acid and a base is taken place because of the transfer of a proton. The stronger the acid, the smaller its
Answer to Problem 63P
Solution:
a)
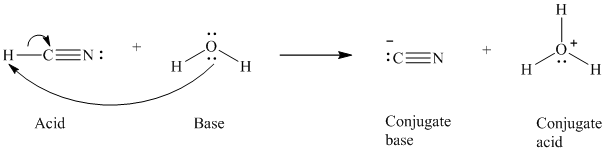
The formal charge on the the oxygen atom is
b)
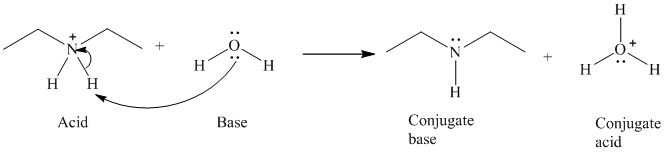
The formal charge on both the the oxygen atom and the nitrogen atom is
c)
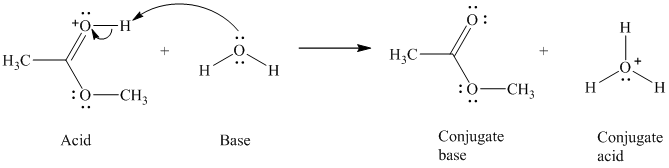 The formal charge on the oxygen atom is
The formal charge on the oxygen atom is
Explanation of Solution
a) The reaction of an acid with water.
In the respective reaction, the water acts as a base. The unshared electron pair of oxygen atom present in water is used to remove the proton from the acid. Water, after accepting the proton, is converted to its conjugate acid, that is, hydronium ion and the acid is converted to its conjugate base.
The curved arrows showing the electron movement is given below:
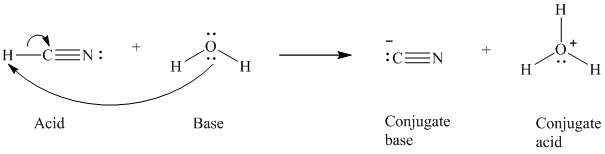
The formula that is used to calculate the electron count on the conjugate base is as follows:
Substitute
The formula that is used to calculate the formal charge on the oxygen atom is as follows:
Substitute
b) The reaction of an acid with water.
The given acid reacts with water. So water acts as a base. The unshared electron pair of the oxygen atom in water is used to remove the proton from the acid. Water, after accepting the proton is converted to its conjugate acid, that is, hydronium ion and the acid is converted to its conjugate base.
The curved arrows showing the electron movement are shown below:
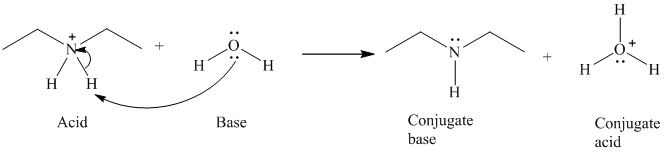
The formula that is used to calculate the electron count on the nitrogen atom is as follows:
Substitute
The formula that is used to calculate the formal charge on the nitrogen atom is as follows:
Substitute
For calculating formal charge on the oxygen atom, recall the electron count formula:
Substitute
Recall the formula for formal charge:
Substitute
c) The given acid reacts with water.
So water acts as a base. The unshared electron pair of the oxygen atom in water is used to remove the proton from the acid. Water, after accepting the proton, is converted to its conjugate acid, that is, hydronium ion and the acid is converted to its conjugate base.
The curved arrows showing the electron movement are shown below:
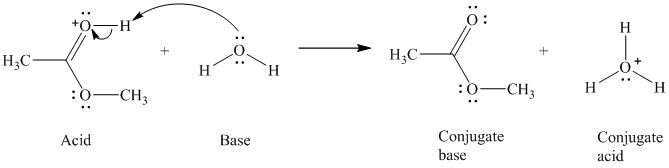
The formula that is used to calculate the electron count on the oxygen is as follows:
Substitute
The formula that is used to calculate the formal charge on the oxygen atom is as follows:
Substitute
Want to see more full solutions like this?
Chapter 1 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
- You will not find “hydroxide” in the stockroom, but you will find sodium hydroxide (NaOH) andpotassium hydroxide (KOH). Lithium hydroxide (LiOH) is expensive and used in spacecraft airfilters since hydroxide reacts with carbon dioxide, and lithium is lighter than sodium or potassium.Cesium and francium hydroxides are very expensive and little used. Is this information consistentwith your answer to the previous question?arrow_forwardThe oxygen atom in acetone possesses ____ unshared pairs and ____ shared pairs of electrons. The number of electrons that belong to oxygen is ____. Oxygen is a Group ____ element. The formal charge on oxygen in acetone is ____.arrow_forwardC4H10O is the formula of diethyl ether. The same group of atoms is attached on either side of the oxygen atom. Draw the Lewis diagram. Diethyl ether was among the first anesthetics used in surgery, but it has been largely replaced by safer anesthetics today.arrow_forward
- Draw the attraction between a water molecule and a molecule of PH3 First, draw one molecule and add the partial charges where needed - use the ΔEN to determine the types of bonds. Then, draw the second molecule so that the δ+ on one molecule lines up across from the δ- on the other. Since we can't draw the molecules here you will answer questions about the drawings that you made on the homework worksheet. PH3 has _________ bonds with a ΔEN = ____________ . PH3 has 4 REDs and is symmetrical/asymmetrical (answer is _________ ) but it is a polar/nonpolar (answer is ___________ ) molecule. Each H has a __________ charge and the P has a __________ charge. H2O has 2 ___________ bonds with a ΔEN = _________ . H2O has 4 REDs and is symmetrical/asymmetrical (answer is________ ) making it a polar/nonpolar (answer is __________) molecule. Each H has a _________ charge and the O has a ________ charge. The strongest possible attractive force between these two molecules is…arrow_forwardThe boiling point of ammonia, NH3 is -33.34 °C while its derivative methylamine, CH3NH2 boiling point is at -6.3 °C both considered weak bases. Provide a detailed explanation on this huge discrepancy of boiling temperature with the help of lewis structure(s) along with your understanding of bonding polarityarrow_forwardAccording to the HONC rule, oxygen atom has a neutral formal charge , when it forms 2 bonds around itarrow_forward
- For conjugate acid-base pair, identify the first species as an acid or a base and the second species as its conjugate base or conjugate acid. In addition, draw Lewis structures for each species, showing all valence electrons and any formal charges. Q) H2CO3, HCO3-arrow_forwardWhich atom bears the formal positive charge in the hydronium ion?arrow_forwardUnshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs.Use the structural formulas below to determine the number of unshared pairs at each designated atom.Be sure your answers are consistent with the formal charges on the formulas. a)The number of unshared pairs at atom a is ___The number of unshared pairs at atom b is ___The number of unshared pairs at atom c is ___ b)The number of unshared pairs at atom a is ___The number of unshared pairs at atom b is ___The number of unshared pairs at atom c is ___arrow_forward
- Write the resonance structure that would result from moving the electrons as the curved arrows indicate. Be sure to include formal charges if needed.arrow_forwardIs maleic acid polar or nonpolar?arrow_forwardHow to easily determikne if a molecule has resonance when comparing two molecules acidities?arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning



