
ORGANIC CHEMISTRY-PRINT COMPANION (LL)
4th Edition
ISBN: 9781119659594
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 1, Problem 69ASP
Interpretation Introduction
Interpretation: The number of
-bonds in the following compound should be determined:
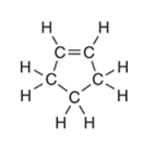
Concept Introduction: The concept of mixing two atomic orbitals that possess almost same energy resulting in formation of new hybridized orbitals that are suitable for pairing the electrons to form
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Considering the structure on the far left as the original resonance structure, which of the subsequent resonance structures (A-D) is implausible.
Explain why you believe the resonance structure to be implausible.
Rank the bonds from most polar to least polar : a. C¬O, C¬F, C¬N b. C¬Cl, C¬I, C¬Br c. H¬O, H¬N, H¬C d. C¬H, C¬C, C¬N
From the given bonds below which bond is shortest?
O a. carbon-oxygen single bond
O b.carbon-nitrogen double bond
c. carbon-oxygen double bond
d.carbon-nitrogen single bond
Chapter 1 Solutions
ORGANIC CHEMISTRY-PRINT COMPANION (LL)
Ch. 1.2 - Prob. 1LTSCh. 1.2 - Prob. 2ATSCh. 1.2 - Prob. 2LTSCh. 1.3 - Prob. 3LTSCh. 1.3 - Prob. 4PTSCh. 1.3 - Prob. 5PTSCh. 1.4 - Prob. 4LTSCh. 1.4 - Prob. 7PTSCh. 1.4 - Prob. 8PTSCh. 1.4 - Prob. 9ATS
Ch. 1.5 - Prob. 5LTSCh. 1.5 - Prob. 10PTSCh. 1.5 - Prob. 11ATSCh. 1.5 - Prob. 12ATSCh. 1.6 - Prob. 6LTSCh. 1.6 - Prob. 14ATSCh. 1.7 - Prob. 7LTSCh. 1.7 - Prob. 17ATSCh. 1.10 - Prob. 18CCCh. 1.10 - Prob. 20CCCh. 1.10 - Prob. 8LTSCh. 1.10 - Prob. 21PTSCh. 1.10 - Nemotin is a compound that was first isolated from...Ch. 1.10 - Prob. 23CCCh. 1.11 - Prob. 9LTSCh. 1.11 - Prob. 24PTSCh. 1.11 - Prob. 25PTSCh. 1.11 - Prob. 26PTSCh. 1.11 - Prob. 27ATSCh. 1.12 - Prob. 10LTSCh. 1.12 - Prob. 29ATSCh. 1.13 - Prob. 11LTSCh. 1.13 - Prob. 31ATSCh. 1 - Prob. 32PPCh. 1 - Prob. 33PPCh. 1 - Prob. 34PPCh. 1 - Prob. 35PPCh. 1 - Prob. 36PPCh. 1 - Prob. 37PPCh. 1 - Prob. 38PPCh. 1 - Prob. 39PPCh. 1 - Prob. 40PPCh. 1 - Prob. 41PPCh. 1 - Prob. 42PPCh. 1 - Prob. 44PPCh. 1 - Prob. 45PPCh. 1 - Prob. 46PPCh. 1 - Prob. 47PPCh. 1 - Prob. 48PPCh. 1 - Prob. 49PPCh. 1 - Prob. 50PPCh. 1 - Prob. 51PPCh. 1 - Prob. 52PPCh. 1 - Prob. 53PPCh. 1 - Prob. 54PPCh. 1 - Nicotine is an addictive substance found in...Ch. 1 - Prob. 56PPCh. 1 - Prob. 57PPCh. 1 - Prob. 59PPCh. 1 - Prob. 63ASPCh. 1 - Prob. 64ASPCh. 1 - Prob. 66ASPCh. 1 - Prob. 69ASPCh. 1 - Prob. 71ASPCh. 1 - Prob. 72ASPCh. 1 - Prob. 75IP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Anacin is an over-the-counter pain reliever that contains aspirin and caffeine. Answer the following questions about each compound. a.What is the molecular formula? b. How many lone pairs are present on heteroatoms? c. Label the hybridization state of each carbon. d. Draw three additional resonance structures.arrow_forwardNow, illustrate the various bond combinations each element can form. Refer to Carbon for the example. Take note that one bond is represented by a line. Nitrogen 3 single b. 1 single, 1 double c. 1 triple Oxygen 2 single b. 1 double Carbon 4 single a. а. а. b. 2 double c. 2 single & 1 double d. 1 single & 1 triple а. a. 4 single bonds а. -C b. b. b.arrow_forwardMCQ 68: The energy which is required to break 1 mole of a given bond is called A. bond energy B. molar energy C. molar bond energy D. bond breaking energyarrow_forward
- A. CHF i. Best Lewis Structure B. HNO (H is connected to one of the O's) i. Best Lewis Structure ii. Electron geometry on the C atom ii. Electron geometry on the N atom iii. Approximate bond angles about the C atom iii. Approximate bond angles around the N atom v. Draw the shape with in and out wedges (as necessary) and dipole arrows around the C atom. v. Draw the shape with in and out wedges (as necessary) and dipole arrows around the N atom. vi. Is the molecule polar or nonpolar? vi. Is the molecule polar or nonpolar?arrow_forwardAnswer the following questions about compound A. a. Label the shortest C-C single bond. b. Label the longest C-C single bond. c. Considering all the bonds, label the shortest C-C bond. d. Label the weakest C-C bond. e. Label the strongest C-H bond. f. Explain why bond (1) and bond (2) are different in length, even though they are both C-C single bonds. (2)arrow_forward2. What type of chemical bonding is present in the following compound? CH3CH2CH2CH2Br O a. A O b. B O C. C O d. D A) Polar covalent B) Hydrogen C) lonic D) polar covalent and non-polar covalentarrow_forward
- MCQ 73: The physical properties of bonding are influenced by bonding between A. atoms B. ions C. molecules D. all of abovearrow_forwardThe molecular geometry consists of __________ a) a nonbonding pair of electrons. b) a single bond. c) a multiple bond. A. a only B. b only C. c only D. b and c E. a, b, and c. My question is: Why is the answer is "D. b and c" instead of "E. a, b, and c." when the "nonbonding pair of electrons" also determine the Molecular Geometry too? For example "Trigonal-planar" electron-group geometry has two molecular geometries such as "trigonal planer" (3 bonds) and "bent" (2 bonds and 1 lone pair electron).arrow_forwardWhat is the bond angel between the N-H bonds in NH3? a. 180 b. 90 c. 109 d. 120arrow_forward
- 3. In a pi bond, A. electron density lies along the internuclear axis. B. electron density lies above and below the internuclear axis. C. electrons are more dense than they are in sigma bonds. D. more than two electrons are involved in the bond.arrow_forwardMCQ 78: A molecule of aluminum chloride (AICI,) is formed by the bond called A. ionic bond B. covalent bond C. co-ordinate bond D. dative bondarrow_forwardAnswer the following questions about compound a. Label the shortest C–C single bond. b. Label the longest C–C single bond. c. Considering all the bonds, label the shortest C–C bond. d. Label the weakest C–C bond. e.Label the strongest C–H bond. f.Explain why bond [1] and bond [2] are different in length, even though they are both C–C single bonds.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY