
a.
Interpretation: The geometry of following structure needs to be predicted:
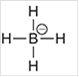
Concept Introduction: The arrangement of electron pairs is identified according to VSEPR (Valence Shell Electron Pair Repulsion) theory wherein all the electron pairs are aligned in such a way that they are at maximum distance from each other. The structure of every molecule in VSEPR is explained by considering the steric number (combination of lone pairs and
b.
Interpretation: The geometry of following structure needs to be predicted:
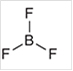
Concept Introduction: The arrangement of electron pairs is identified according to VSEPR (Valence Shell Electron Pair Repulsion) theory wherein all the electron pairs are aligned in such a way that they are at maximum distance from each other. The structure of every molecule in VSEPR is explained by considering the steric number (combination of lone pairs and
c.
Interpretation: The geometry of following structure needs to be predicted:
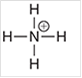
Concept Introduction: The arrangement of electron pairs is identified according to VSEPR (Valence Shell Electron Pair Repulsion) theory wherein all the electron pairs are aligned in such a way that they are at maximum distance from each other. The structure of every molecule in VSEPR is explained by considering the steric number (combination of lone pairs and
d.
Interpretation: The geometry of following structure needs to be predicted:
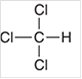
Concept Introduction: The arrangement of electron pairs is identified according to VSEPR (Valence Shell Electron Pair Repulsion) theory wherein all the electron pairs are aligned in such a way that they are at maximum distance from each other. The structure of every molecule in VSEPR is explained by considering the steric number (combination of lone pairs and
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
ORGANIC CHEMISTRY-PRINT COMPANION (LL)
- Draw diagrams similar to Figure 16.1 to illustrate hydrogen bonding between the following compounds: a.CH3CH2NHCH3 and H2O b.andarrow_forwardamong compound that are hydrophobic one would find a. electroltyes b. non-polar covalent compounds c. polar compounds d. ionsarrow_forwardF. F C. 2. F F draw structurearrow_forward
- Determine which of these best describes the given structure: A. Aromatic B. Antiaromatic C. Nonaromatic - not fully conjugated D. Nonaromatic - nonHuckel E. Nonaromatic - not planararrow_forwardUse the References to access important values if needed for this question. Classify each of the following hydrocarbons as saturated or unsaturated. H. H. H H. H. H- H H H. H Previous Next Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forwardUse the References to access important values if needed for this question. Use the following Lewis diagram for ethyl methyl ether to answer the questions: Remember that geometry refers to the geometry defined by the atoms, not the electron pairs. The geometry about atom O¡ is The ideal value of the O-C-C angle at atom C2 is |degrees. The geometry about atom C3 is Mastered Submit Answer Retry Entire Group 8 more group attempts remaining Previous Nextarrow_forward
- The term POLAR as in POLAR MOLECULE refers to...: A. Molecules that are present in magnets and which give that material magnetic properties B. Molecules that are composed of asymmetrical hydrophobic molecules so that they have a lipid pole. C. Molecules in which the atomic components are arranged asymmetrically to give opposite charges on the ends.arrow_forwardThe compound that has the greatest polarity is: a. CH3CH2OCH2CH3 b. CH3CH2CH2CH2CH3 c. CH3CH2CH2CH2CH2Cl d. CH3CH2CH2CH2CH2OHarrow_forwardIn terms of the bonds present, explain why acetic acid, CH3CO2H, contains two distinct types of carbon-oxygen bonds, whereas the acetate ion, formed by loss of a hydrogen ion from acetic acid, only contains one type of carbon oxygen bond. The skeleton structures of these species are shown:arrow_forward
- Name d. And e. Organic molecules.arrow_forward3. The functional group in compound below is called... (Please see attached image) A. ketone B. acid C. ester D. amine E. None of the others F. amidearrow_forwardUse the following Lewis diagram for 1-propanol to answer the questions: 3. Remember that geometry refers to the geometry defined by the atoms, not the electron pairs. The geometry about atom C, is The ideal value of the C-C-C angle at atom C2 is degrees. The geometry about atom 0z is Submit Answer Retry Entire Group 9 more group attempts remaining Previous Next Email Instructor Save and Exit Cengage Learning | Cengage Technical Supportarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax




