
Concept explainers
Stalevo is the trade name for a medication used fssor Parkinson’s disease, which contains L-dopa, carbidopa, and entacapone.
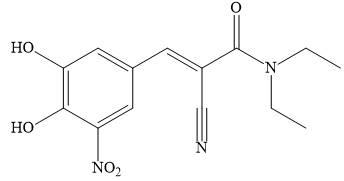
a. Draw a Lewis structure for entacapone.
b. Which
c. Which
d. Which
e. Which
f. Use curved arrows to draw a resonance structure that is an equal contributor to the resonance hybrid.
g. Use curved arrows to draw a resonance structure that is a minor contributor to the resonance hybrid.
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
ORG CHEM (CUSTOM F/IA CHE 211/212 WC)
- The chemistry of the nitrite ion and HNO2: (a) Two resonance structures are possible for NO2. Draw these structures, and then find the formal charge on each atom in each resonance structure. (b) In forming the acid HNO2 an H+ ion attaches to the O atom and not the N atom of NO2. Explain why you would predict this result. (c) Two resonance structures are possible for HNO2. Draw these structures, and then find the formal charge on each atom in each resonance structure. Is either of these structures strongly preferred over the other?arrow_forwardAcrolein is used to make plastics. Suppose this compound can be prepared by inserting a carbon monoxide molecule into the CH bond of ethylene. (a) Which is the stronger carbon-carbon bond in acrolein? (b) Which is the longer carbon-carbon bond in acrolein? (c) Is ethylene or acrolein polar? (d) Use bond dissociation enthalpies to predict whether the reaction of CO with C2H4 to give acrolein is endothermic or exothermic.arrow_forwardPhosgene, Cl2CO, is a highly toxic gas that was used as a weapon in World War 1. Using the bond dissociation enthalpies in Table 8.8, estimate the enthalpy change for the reaction of carbon monoxide and chlorine to produce phosgene. CO(g) + Cl2(g) Cl2CO(g)arrow_forward
- Compare the nitrogen-nitrogen bond in hydrazine, H2NNH2, with that in laughing gas, N2O. In which molecule is the nitrogen-nitrogen bond shorter? In which is the bond stronger?arrow_forwardHow does the bond energy of HCl(g) differ from the standard enthalpy of formation of HCl(g)?arrow_forwardWhich type of bond exists in each compound? KCl a. polar covalent bonds b. ionic bonds c. nonpolar covalent bonds BCl3 a. ionic bonds b. polar covalent bonds c. nonpolar covalent bonds P4 a. nonpolar covalent bonds b. ionic bonds c. polar covalent bonds Br2 a. ionic bonds b. polar covalent bonds c. nonpolar covalent bonds CO a. polar covalent bonds b. ionic bonds c. nonpolar covalent bonds SO2 a. nonpolar covalent bonds b. ionic bonds c. polar covalent bondsarrow_forward
- a. In the following group, which element is the most electronegative? Na, Cs, Be Be is the most electronegative. is the most electronegative. OCs is the most electronegative. b. In the following group, which element is the least electronegative? Na, Cs, Be O Be is the least electronegative. is the least electronegative. OCs is the least electronegative. c. In the following group, which element is the most electronegative? K, Ca, Br O Ca is the most electronegative. OK is the most electronegative. O Br is the most electronegative. d. In the following group, which element is the least electronegative? K, Ca, Br O Ca is the least electronegative. OK is the least electronegative. Br is the least electronegative.arrow_forwardA. Complete the table Bond formation according What happened to the e's? Type of compound formed Given to atoms | 1, polar bond 2. nonpolar bond 3. ionic bond B. Draw the chemical bond of the following: [Lewis Dot] 1. Phosphorous (P-Group VA) + Chlorine (CI-Group VIIA) 2. Aluminum (Al 13) + Sulfur (S 16) 3. Nitrogen (N-Group VA) + Flourine (F-Group VIIA) 4. Potassium (K19) + Oxygen (08) 5. Astatine (At-Group VIIA) Astatine (At-Group VIIA)arrow_forwardb. There is one additional resonance structure. NH₂ c. There is a total of five resonance structures (including the original structure). : OHarrow_forward
- 2. Avogadro does not "waste" his time drawing a Lewis structure before determining the shape of PF3. He thinks that the shape of PF3 must be trigonal planar because there are three fluorine atoms bonded to the central phosphorus atom. a. Draw the Lewis structure for PF3. b. Was Avogadro's answer for the shape of a PF3 molecule correct? Explain c. Why is it important to draw the Lewis structure for a molecule before identifying the shape of the molecule?arrow_forward2. Avogadro does not "waste" his time drawing a Lewis structure before determining the shape of PF3. He thinks that the shape of PF3 must be trigonal planar because there are three fluorine atoms bonded to the central phosphorus atom. a. Draw the Lewis structure for PF3. b. Was Avogadro's answer for the shape of a PF3 molecule correct? Explain c. Why is it important to draw the Lewis structure for a molecule before identifying the shape of the molecule? 3. Draw the Lewis structure of ozone, O3. Describe why ozone has a bent shape instead of a linear shape.arrow_forwardDraw the Lewis structure for NO2, including any valid resonance structures. Which of the following statements is TRUE? O a. The nitrite ion contains two N-O single bonds. O b. The nitrite ion contains two N=O double bonds. Oc. The nitrite ion contains two N-O bonds that are equivalent to 1 bonds. O d. The nitrite ion contains one N-O single bond and one N=O double bond. O e. None of the other choices is correctarrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





