
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 85GQ
Acrolein is used to make plastics. Suppose this compound can be prepared by inserting a carbon monoxide molecule into the C—H bond of ethylene.
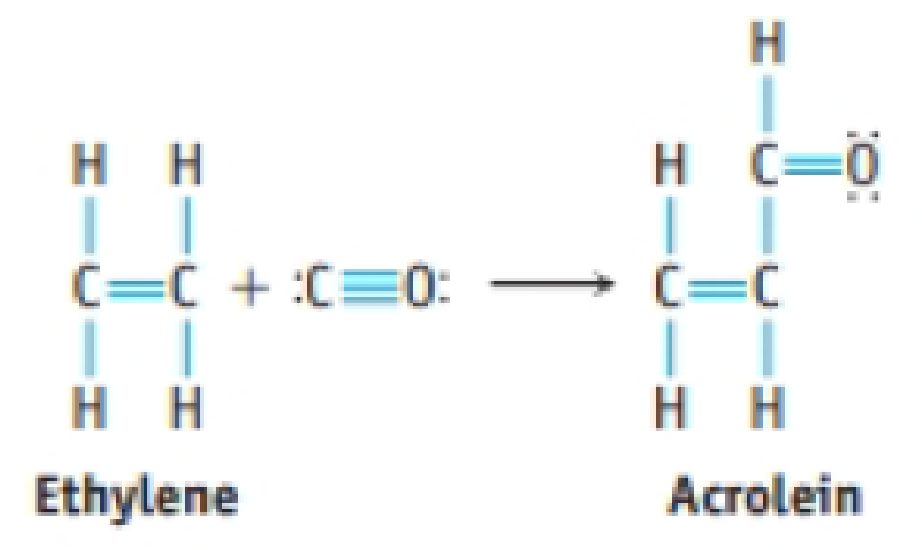
- (a) Which is the stronger carbon-carbon bond in acrolein?
- (b) Which is the longer carbon-carbon bond in acrolein?
- (c) Is ethylene or acrolein polar?
- (d) Use bond dissociation enthalpies to predict whether the reaction of CO with C2H4 to give acrolein is endothermic or exothermic.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Aluminum oxide (Al₂ O₃) is a widely used industrial abrasive(emery, corundum), for which the specific application depends onthe hardness of the crystal. What does this hardness imply about the magnitude of the lattice energy? Would you have predictedfrom the chemical formula that Al₂ O₃ is hard? Explain.
If a C-O bond length is 1.66 pm and a C=O bond length is 1.52 pm, how long would the carbon oxygen bonds in the carbonate ion be?
Based on average bond enthalpies, would you expect a photon capable ofdissociating a C¬Cl bond to have sufficient energy to dissociate a C¬Br bond?
Chapter 8 Solutions
Chemistry & Chemical Reactivity
Ch. 8.2 - Draw Lewis electron dot structures for CH3Cl...Ch. 8.2 - Prob. 8.2CYUCh. 8.2 - Prob. 8.3CYUCh. 8.2 - Prob. 8.4CYUCh. 8.3 - Prob. 8.5CYUCh. 8.4 - Draw resonance structures for the bicarbonate ion,...Ch. 8.5 - Sketch the Lewis structures for CIF2+ and CIF2....Ch. 8.6 - What is the shape of the dichloromethane (CH2C12)...Ch. 8.6 - Give the electron-pair geometry and molecular...Ch. 8.6 - Draw the Lewis structure for lCl2, and then decide...
Ch. 8.7 - For each of the following pairs of bonds, decide...Ch. 8.7 - Draw the resonance structures for SCN. What are...Ch. 8.8 - For each of the following molecules, decide...Ch. 8.8 - The electrostatic potential surface for SOCl2 is...Ch. 8.9 - Using the bond dissociation enthalpies in Table...Ch. 8.10 - Prob. 1.1ACPCh. 8.10 - Do any of the atoms in an ibuprofen molecule have...Ch. 8.10 - What is the most polar bond in the molecule?
Ch. 8.10 - Prob. 1.4ACPCh. 8.10 - Prob. 1.5ACPCh. 8.10 - Prob. 1.6ACPCh. 8.10 - Are there any 120° bond angles in ibuprofen? Any...Ch. 8.10 - Prob. 1.8ACPCh. 8.10 - Prob. 2.2ACPCh. 8.10 - Calculate the difference in electronegativity...Ch. 8.10 - Predict the bond dissociation enthalpy for a...Ch. 8.10 - Prob. 3.3ACPCh. 8 - Give the periodic group number and number of...Ch. 8 - Give the periodic group number and number of...Ch. 8 - For elements in Groups 4A-7A of the periodic...Ch. 8 - Prob. 4PSCh. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Show all possible resonance structures for each of...Ch. 8 - Show all possible resonance structures for each of...Ch. 8 - Prob. 11PSCh. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Determine the formal charge on each atom in the...Ch. 8 - Determine the formal charge on each atom in the...Ch. 8 - Determine the formal charge on each atom in the...Ch. 8 - Determine the formal charge on each atom in the...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Prob. 18PSCh. 8 - Prob. 19PSCh. 8 - The following molecules or ions all have three...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Give approximate values for the indicated bond...Ch. 8 - Give approximate values for the indicated bond...Ch. 8 - Phenylalanine is one of the natural amino acids...Ch. 8 - Acetylacetone has the structure shown here....Ch. 8 - For each pair of bonds, indicate the more polar...Ch. 8 - For each of the bonds listed below, tell which...Ch. 8 - Urea, (NH2)2CO, is used in plastics and...Ch. 8 - Considering both formal charges and bond...Ch. 8 - Considering both formal charge and bond...Ch. 8 - Three resonance structures are possible for...Ch. 8 - Three resonance structures are possible for the...Ch. 8 - Compare the electron dot structures of the...Ch. 8 - Compare the electron dot structures of the...Ch. 8 - The chemistry of the nitrite ion and HNO2: (a) Two...Ch. 8 - Draw the resonance structures for the formate ion,...Ch. 8 - Prob. 39PSCh. 8 - Consider the following molecules: (a) CH4 (b)...Ch. 8 - Which of the following molecules is(are) polar?...Ch. 8 - Prob. 42PSCh. 8 - Give the bond order for each bond in the following...Ch. 8 - Prob. 44PSCh. 8 - In each pair of bonds, predict which is shorter....Ch. 8 - In each pair of bonds, predict which is shorter....Ch. 8 - Prob. 47PSCh. 8 - Compare the carbon-oxygen bond lengths in the...Ch. 8 - Consider the carbon-oxygen bond in formaldehyde...Ch. 8 - Compare the nitrogen-nitrogen bond in hydrazine,...Ch. 8 - Ethanol can be made by the reaction of ethylene...Ch. 8 - Methanol can be made by partial oxidation of...Ch. 8 - Hydrogenation reactions, which involve the...Ch. 8 - Phosgene, Cl2CO, is a highly toxic gas that was...Ch. 8 - The compound oxygen difluoride is quite reactive,...Ch. 8 - Oxygen atoms can combine with ozone to form...Ch. 8 - Prob. 57GQCh. 8 - Prob. 58GQCh. 8 - Which of the following compounds or ions do not...Ch. 8 - Prob. 60GQCh. 8 - Draw resonance structures for the formate ion,...Ch. 8 - Prob. 62GQCh. 8 - Prob. 63GQCh. 8 - What is the principle of electroneutrality? Use...Ch. 8 - Prob. 65GQCh. 8 - Draw resonance structures for the SO2 molecule,...Ch. 8 - What are the orders of the NO bonds in NO2 and...Ch. 8 - Which has the greater ONO bond angle, NO2 or NO2+?...Ch. 8 - Compare the FClF angles in CIF2+ and ClF2. Using...Ch. 8 - Draw an electron dot structure for the cyanide...Ch. 8 - Draw the electron dot structure for the sulfite...Ch. 8 - Dinitrogen monoxide, N2O, can decompose to...Ch. 8 - The equation for the combustion of gaseous...Ch. 8 - The cyanate ion, OCN, has the least...Ch. 8 - Vanillin is the flavoring agent in vanilla extract...Ch. 8 - Explain why (a) XeF2 has a linear molecular...Ch. 8 - The formula for nitryl chloride is ClNO2 (in which...Ch. 8 - Hydroxyproline is a less-common amino acid. (a)...Ch. 8 - Amides are an important class of organic...Ch. 8 - Prob. 81GQCh. 8 - The molecule shown here. 2-furylmelhanethiol, is...Ch. 8 - Dihydroxyacetone is a component of quick-tanning...Ch. 8 - It is possible to draw three resonance structures...Ch. 8 - Acrolein is used to make plastics. Suppose this...Ch. 8 - Molecules in space: (a) In addition to molecules...Ch. 8 - 1,2-Dichloroethylene can be synthesized by adding...Ch. 8 - The molecule pictured below is epinephrine, a...Ch. 8 - You are doing an experiment in the laboratory and...Ch. 8 - Prob. 90ILCh. 8 - A paper published in the research Journal Science...Ch. 8 - Uracil is one of the bases in RNA, a close...Ch. 8 - Guanine is present in both DNA and RNA. (a) What...Ch. 8 - Prob. 94ILCh. 8 - Prob. 95SCQCh. 8 - Prob. 96SCQCh. 8 - Bromine-containing species play a role in...Ch. 8 - Acrylamide, H2C=CHCONH2, is a known neurotoxin and...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write all resonance structures of chlorobenzene, C6H5Cl, a molecule with the same cyclic structure as benzene. In all structures, keep the CCl bond as a single bond. Which resonance structures are the most important?arrow_forwardUsing the bond dissociation enthalpies in Table 8.8, estimate the enthalpy of combustion of gaseous methane, CH4, to give water vapor and carbon dioxide gas.arrow_forwardExplain the decomposition of nitroglycerin in terms of relative bond enthalpies.arrow_forward
- Define the term lattice energy. Why, energetically, do ionic compounds form? Fig. 3-8 illustrates the energy changes involved in the formation of MgO(s) and NaF(s). Why is the lattice energy of MgO(s) so different from that of NaF(s)? The magnesium oxide is composed of Mg2+ and O2 ions. Energetically, why does Mg2+O2 form and not Mg+O? Why doesnt Mg3+O3 form?arrow_forwardWhich atom in the C-F bond has a partial negative charge (δ⁻)?arrow_forwardAcetylene 1C2H22 and nitrogen 1N22 both contain a triplebond, but they differ greatly in their chemical properties.(a) Write the Lewis structures for the two substances. (b) Byreferring to Appendix C, look up the enthalpies of formationof acetylene and nitrogen. Which compound is more stable?(c) Write balanced chemical equations for the completeoxidation of N2 to form N2O51g2 and of acetylene to formCO21g2 and H2O1g2. (d) Calculate the enthalpy of oxidationper mole for N2 and for C2H2 (the enthalpy of formationof N2O51g2 is 11.30 kJ>mol). (e) Both N2 and C2H2 possesstriple bonds with quite high bond enthalpies (Table 8.3).Calculate the enthalpy of hydrogenation per mole for bothcompounds: acetylene plus H2 to make methane, CH4;nitrogen plus H2 to make ammonia, NH3.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
INTRODUCTION TO MOLECULAR QUANTUM MECHANICS -Valence bond theory - 1; Author: AGK Chemistry;https://www.youtube.com/watch?v=U8kPBPqDIwM;License: Standard YouTube License, CC-BY