
(a)
Interpretation:
The reaction,
Concept introduction:
A redox reaction is a type of reaction in which one reacting species gets oxidize and other reacting species is reduced. The exchange of electron takes place between the reacting species. Water molecules and protons can be used to
Answer to Problem 10.26P
The reaction,
Explanation of Solution
The given reaction is shown below.
The reactant and products with an oxidation number of atoms are shown below.
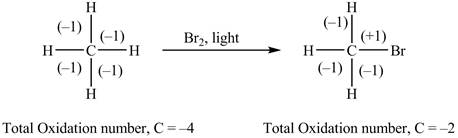
Figure 1
The oxidation number of reactant is lower than the oxidation number of the product. Therefore, the reaction is an oxidation reaction.
The number of electrons transfer in the reaction is given by the expression as shown below.
Where,
•
•
Substitute the values of
Therefore, the number of electrons transferred in the reaction is
The number of electrons transferred in the oxidation reaction is
(b)
Interpretation:
The given reaction is to be classified as an oxidation, a reduction, or neither. The number of electrons transferred in the reaction is to be stated when the corresponding reaction is oxidation or reduction.
Concept introduction:
A redox reaction is a type of reaction in which one reacting species gets oxidize and other reacting species is reduced. The exchange of electron takes place between the reacting species. Water molecules and protons can be used to balance a redox
Answer to Problem 10.26P
The given reaction is classified as oxidation reaction. The number of electrons transferred in the reaction is
Explanation of Solution
The given reaction is shown below.
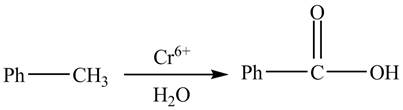
Figure 2
The reactant and products with oxidation number of atoms are shown below.
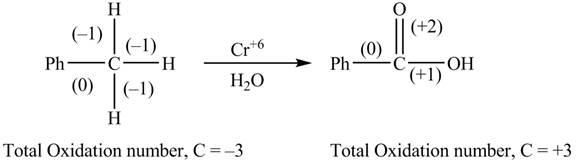
Figure 3
The oxidation number of reactant is lower than the oxidation number of the product. Therefore, the reaction is an oxidation reaction.
The number of electrons transfer in the reaction is given by the expression as shown below.
Where,
•
•
Substitute the values of
Therefore, the number of electrons transferred in the reaction is
The number of electrons transferred in the oxidation reaction is
(c)
Interpretation:
The reaction,
Concept introduction:
A redox reaction is a type of reaction in which one reacting species gets oxidize and other reacting species is reduced. The exchange of electron takes place between the reacting species. Water molecules and protons can be used to balance a redox chemical reaction in the acidic medium.
Answer to Problem 10.26P
The reaction,
Explanation of Solution
The given reaction is shown below.
The reactant and products with oxidation number of atoms are shown below.
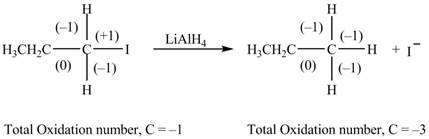
Figure 4
The oxidation number of reactant is higher than the oxidation number of the product. Therefore, the reaction is the reduction reaction.
The number of electrons transfer in the reaction is given by the expression as shown below.
Where,
•
•
Substitute the values of
Therefore, the number of electrons transferred in the reaction is
The number of electrons transferred in the reduction reaction is
(d)
Interpretation:
The given reaction is to be classified as an oxidation, a reduction, or neither. The number of electrons transferred in the reaction is to be stated when the corresponding reaction is oxidation or reduction.
Concept introduction:
A redox reaction is a type of reaction in which one reacting species gets oxidize and other reacting species is reduced. The exchange of electron takes place between the reacting species. Water molecules and protons can be used to balance a redox chemical reaction in the acidic medium.
Answer to Problem 10.26P
The given reaction is to be classified as oxidation reaction. The number of electrons transferred in the reaction is
Explanation of Solution
The given reaction is shown below.
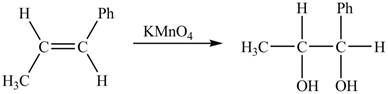
Figure 5
The reactant and products with oxidation number of atoms are shown below.
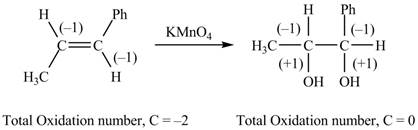
Figure 6
The oxidation number of reactant is lower than the oxidation number of the product. Therefore, the reaction is an oxidation reaction.
The number of electrons transfer in the reaction is given by the expression as shown below.
Where,
•
•
Substitute the values of
Therefore, the number of electrons transferred in the reaction is
The number of electrons transferred in the oxidation reaction is
(e)
Interpretation:
The given reaction is to be classified as an oxidation, a reduction, or neither. The number of electrons transferred in the reaction is to be stated when the corresponding reaction is oxidation or reduction.
Concept introduction:
A redox reaction is a type of reaction in which one reacting species gets oxidize and other reacting species is reduced. The exchange of electron takes place between the reacting species. Water molecules and protons can be used to balance a redox chemical reaction in the acidic medium.
Answer to Problem 10.26P
The given reaction is to be classified as oxidation reaction. The number of electrons transferred in the reaction is
Explanation of Solution
The given reaction is shown below.
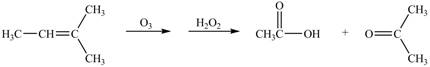
Figure 7
The reactant and products with oxidation number of atoms are shown below.
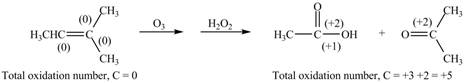
Figure 8
The oxidation number of reactant is lower than the oxidation number of product. Therefore, the reaction is oxidation reaction.
The number of electrons transfer in the reaction is given by the expression as shown below.
Where,
•
•
Substitute the values of
Therefore, the number of electrons transferred in the reaction is
The number of electrons transferred in the oxidation reaction is
(f)
Interpretation:
The given reaction is to be classified as an oxidation, a reduction, or neither. The number of electrons transferred in the reaction is to be stated when the corresponding reaction is oxidation or reduction.
Concept introduction:
A redox reaction is a type of reaction in which one reacting species gets oxidize and other reacting species is reduced. The exchange of electron takes place between the reacting species. Water molecules and protons can be used to balance a redox chemical reaction in the acidic medium.
Answer to Problem 10.26P
The given reaction is to be classified as neither
Explanation of Solution
The given reaction is shown below.
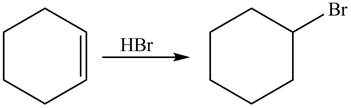
Figure 9
The reactant and products with an oxidation number of atoms are shown below.
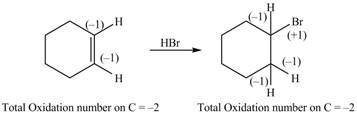
Figure 10
The oxidation number of reactant is equal to the oxidation number of the product. Therefore, the reaction is neither oxidation reaction nor reduction reaction.
The compound neither oxidized nor reduced.
(g)
Interpretation:
The given reaction is to be classified as an oxidation, a reduction, or neither. The number of electrons transferred in the reaction is to be stated when the corresponding reaction is oxidation or reduction.
Concept introduction:
A redox reaction is a type of reaction in which one reacting species gets oxidize and other reacting species is reduced. The exchange of electron takes place between the reacting species. Water molecules and protons can be used to balance a redox chemical reaction in the acidic medium.
Answer to Problem 10.26P
The given reaction is to be classified as a reduction reaction. The number of electrons transferred in the reaction is
Explanation of Solution
The given reaction is shown below.
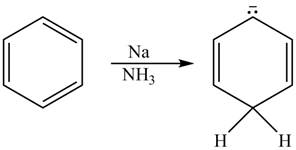
Figure 11
The reactant and products with oxidation number of atoms are shown below.
\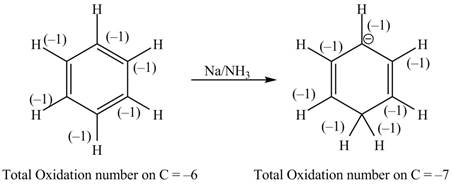
Figure 12
The oxidation number of reactant is higher than the oxidation number of the product. Therefore, the reaction is reduction reaction.
The number of electrons transfer in the reaction is given by the expression as shown below.
Where,
•
•
Substitute the values of
Therefore, the number of electrons transferred in the reaction is
The number of electrons transferred in the reduction reaction is
Want to see more full solutions like this?
Chapter 10 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- A rebreathing gas mask contains potassium superoxide, KO2, which reacts with moisture in the breath to give oxygen. 4KO2(s)+2H2O(l)4KOH(s)+3O2(g) Estimate the grams of potassium superoxide required to supply a persons oxygen needs for one hour. Assume a person requires 1.00 102 kcal of energy for this time period. Further assume that this energy can be equated to the heat of combustion of a quantity of glucose, C6H12O6, to CO2(g) and H2O(l). From the amount of glucose required to give 1.00 102 kcal of heat, calculate the amount of oxygen consumed and hence the amount of KO2 required. The ff0 for glucose(s) is 1273 kJ/mol.arrow_forwardThe carbon dioxide exhaled in the breath of astronauts is often removed from the spacecraft by reaction with lithium hydroxide 2LiOH(s)+CO2(g)Li2CO3(s)+H2O(l) Estimate the grams of lithium hydroxide required per astronaut per day. Assume that each astronaut requires 2.50 103 kcal of energy per day. Further assume that this energy can be equated to the heat of combustion of a quantity of glucose, C6H12O6, to CO2(g) and H2O(l). From the amount of glucose required to give 2.50 103 kcal of heat, calculate the amount of CO2 produced and hence the amount of LiOH required. The H for glucose(s) is 1273 kJ/mol.arrow_forwardEthanol, C2H5OH, is a gasoline additive that can be produced by fermentation of glucose. C6H12O62C2H5OH+2CO2 (a) Calculate the mass (g) of ethanol produced by the fermentation of 1.000 lb glucose. (b) Gasohol is a mixture of 10.00 mL ethanol per 90.00 mL gasoline. Calculate the mass (in g) of glucose required to produce the ethanol in 1.00 gal gasohol. Density of ethanol = 0.785 g/mL. (c) By 2022, the U. S. Energy Independence and Security Act calls for annual production of 3.6 1010 gal of ethanol, no more than 40% of it produced by fermentation of corn. Fermentation of 1 ton (2.2 103 lb) of corn yields approximately 106 gal of ethanol. The average corn yield in the United States is about 2.1 105 lb per 1.0 105 m2. Calculate the acreage (in m2) required to raise corn solely for ethanol production in 2022 in the United States.arrow_forward
- What coefficients are needed to balance the equation for the complete combustion of methane? Enter the coefficients in the order CH4, O2, CO2, and H2O, respectively.arrow_forwardSyngas is a mixture of carbon monoxide, carbon dioxide, and water vapor that is used for making fertilizer, methanol, and other products. It is produced by heating fossil fuels under low-oxygen conditions to promote incomplete combustion. Write a balanced chemical equation, using the simplest ratio of integer coefficients, for the complete combustion of the gasoline component cyclopentane, C5H10 (l). Write a balanced chemical equation, using the simplest ratio of integer coefficients, for the incomplete combustion of cyclopentane with 20% of the carbon from cyclopentane appearing as CO(g) in the products.arrow_forwardWhat is the sum of coefficients from the following reaction (both products and reactants)? X(OH)5 + Heat --->arrow_forward
- Write and balance the reaction for the complete combustion of heptane, C7H16.arrow_forwarda student carries out this chemical reaction in a beaker Zn+CuCl2 -> Cu+ZnCl2 after the reaction has come to completion, what should the student expect to find in the beaker ?arrow_forward3. In a chemical reaction, energy is lost in the form of heat A.True B. Falsearrow_forward
- What is the kinetic energy of a baseball with a mass of 117.7 g traveling at a speed of 1.377 m/s?arrow_forwardWhich of the following represents the generic form of a synthesis reaction? A) A + BX → AX + B B) A + B → AB C) AB → A + B D) AX + BY → AY + BXarrow_forwardA student is preparing a solution of sucrose (C12H22O11) at a specific concentration. They are doing this by weighing out sucrose on a scale, placing it in a volumetric flask, dissolving it in water, and then diluting it up to the mark on the volumetric flask with water. How would the experimental (actual) molar concentration for C12H22O11 differ from the calculated concentration (higher, lower, or the same) given the following scenarios? Explain. (Hint: Compare the calculated concentrations to experimental concentrations). c) When preparing the diluted solution, a student used the pipet bulb to blow out the last few drops in the pipet into the solution.arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





