
Concept explainers
(a)
Interpretation:
The alcohol from which the compound
Concept introduction:
Primary and secondary alcohols can be oxidized into
Answer to Problem 10.31P
The
Explanation of Solution
Primary and secondary alcohols can be oxidized into aldehydes and ketones using
The compound
The carboxylic acid,
The alcohol from which the compound
(b)
Interpretation:
The alcohol from which the compound
Concept introduction:
Primary and secondary alcohols can be oxidized into aldehydes and ketones using
Answer to Problem 10.31P
The
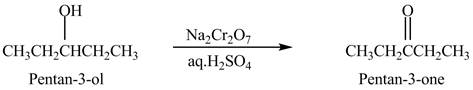
Explanation of Solution
Primary and secondary alcohols are oxidized into aldehydes and ketones using
The secondary alcohols are converted into ketones on oxidation.
The compound
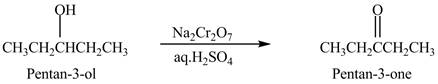
Figure 1
The ketone
The alcohol from which the compound
(c)
Interpretation:
The alcohol from which the compound
Concept introduction:
Primary and secondary alcohols can be oxidized into aldehydes and ketones using
Answer to Problem 10.31P
The
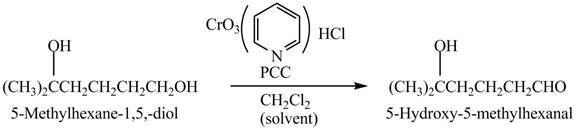
Explanation of Solution
Primary and secondary alcohols can be oxidized into aldehydes and ketones using
The primary alcohols are converted into aldehydes under anhydrous conditions.
The compound

Figure 2
The aldehyde,
The alcohol from which the compound
(d)
Interpretation:
The alcohol from which the compound
Concept introduction:
Primary and secondary alcohols can be oxidized into aldehydes and ketones using
Answer to Problem 10.31P
The
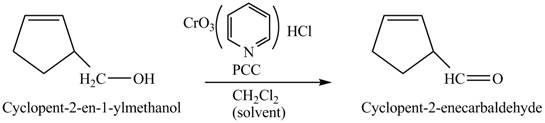
Explanation of Solution
Primary and secondary alcohols can be oxidized into aldehydes and ketones using
The primary alcohols are oxidized into aldehydes under anhydrous conditions.
The compound
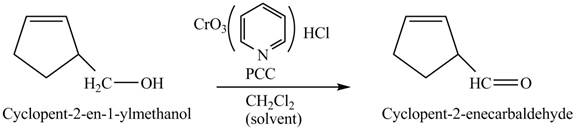
Figure 3
The compound
The alcohol from which the compound
Want to see more full solutions like this?
Chapter 10 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- Hydrocarbon X has the formula C6H12.X reacts with one molar equivalent of hydrogen in the presence of a palladium catalyst to form a product having 12 primary hydrogens.Treatment of X with ozone followed by zinc in aqueous acid gives a mixture two aldehydes.What is the structure of X?arrow_forwardUnlike ethylene glycol, propylene glycol (propane-1,2-diol) is nontoxic because it oxidizes to a common metabolic intermediate. Give the structures of the biological oxidationproducts of propylene glycol.arrow_forwardDescribe the procedures on how will you prepare an alcohol from an alkene.arrow_forward
- Diazomethane, CH2N2, is used in the organic chemistry laboratory despite its danger because it produces very high yields and is selective for reaction with carboxylic acids. Write the products of the following reactions.arrow_forwardWrite chemical equations when (i) ethyl chloride is treated with alcoholic KOH. (ii) chlorobenzene is treated with CH3Cl in the presence of anhydrous AlCl3.arrow_forwardWrite structural formulas for all ketones with the molecular formula C6H12O and give each its IUPAC name. Which of these ketones are chiral?arrow_forward
- Three constitutional isomers of molecular formula C 5H 8O can be converted to 1-pentanol (CH 3CH 2CH 2CH 2CH 2OH) on treatment with two equivalents of H 2 in the presence of a Pd catalyst. Draw the structures of the three possible compounds, all of which contain a carbonyl grouparrow_forwardAn unknown hydrocarbon A with the formula C6H12 reacts with 1 molar equivalent of H2 over a palladium catalyst. Hydrocarbon A also reacts with OsO4 to give diol B. When oxidized with KMnO4 in acidic solution, A gives two fragments. One fragment is propanoic acid, CH3CH2CO2H, and the other fragment is ketone C. What are the structures of A, B, and C? Write all reactions, and show your reasoning.arrow_forwardAn organic compound A of unknown structure was found to have a molecular formula C8H16. When A was poured in water and heated, compound B having a molecular formula C8H18O was formed. B upon heating with sulfuric acid was converted to C as the major product which is identical to A. Ozonolysis of C gave one molecule each of two different products D and E, both having a molecular formula C4H8O. Write the reactions involved and determine the structure of A,B,C,D and E.arrow_forward
- Which alcohols can be prepared as a single product by hydroboration–oxidation of an alkene? Which alcohols can be prepared as a singleproduct by the acid-catalyzed addition of H2O to an alkene?arrow_forwardAlcohol A (C10H18O) is converted to a mixture of alkenes B and C on being heated with potassium hydrogen sulfate (KHSO4). Catalytic hydrogenation of B and C yields the same product. Assuming that dehydration of alcohol A proceeds without rearrangement, deduce the structures of alcohol A and alkene C.arrow_forwardWrite structural formulas for all aldehydes with the molecular formula C6H12O and give each its IUPAC name. Which of these aldehydes are chiralarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





