
ORGANIC CHEMISTRY (LL) W/WILEYPLUS NEXT
12th Edition
ISBN: 9781119664635
Author: Solomons
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 19P
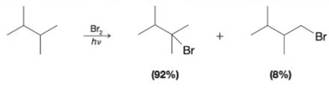
Explain the relative distribution of produces below using reaction energy diagrams for the hydrogen abstraction step that leads to each product. (The rare-determining seep in radical halogenation is the hydrogen abstraction step.) In energy diagrams for the two pathways, show die relative energies of the transition scares and of the alkyl radical intermediate char results in each case.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
] The reaction of 2-bromopropane and sodium ethoxide in ethanol reacts 6.7 times faster than 2-bromo-1-deuteriopropane under the same conditions. Explain what mechanism this data is consistent with, and why
The high reactivity of alkyl halides can be explained in terms of nature of C-X bond which is highly polarized
covalent bond due to large difference in the electronegativities of carbon and halogen atom. This polarity
is responsible for the nucleophilic substitution reactions of alkyl halides which mostly occur by Swa and Swa
mechanisms. Sy reaction is a two-step process and in the first step, R-X ionizes to give carbocation (slow
process). In the second step, the nucleophile attacks the carbocation from either side to form the product
(fast process). In Swi reaction, there can be racemization and inversion. Swi reaction is favored by heavy
(bulky) groups on the carbon atom attached to halogens. i.e., R,C-X>R;CH-X>R-CH,X>CH,X. In Sna reaction,
the strong nucleophile OH attacks from the opposite side of the chlorine atom to give an intermediate
(transition state) which breaks to yield the product (alcohol) and leaving (X) group. The alcohol has a
configuration opposite to that of the…
Heterocyclic compounds plays an important role in our daily life. They are mainly used in
pharmaceutical and agrochemical products to name a few.
3. It is required to introduce a halogen group to a five membered ring, thiophene. Discuss
the reaction mechanism involved in the reaction by selecting a suitable halogen group and
analyze why a particular substituted product obtained after the reaction is predominant
over the other possible product(s) with the help of reactions.
Chapter 10 Solutions
ORGANIC CHEMISTRY (LL) W/WILEYPLUS NEXT
Ch. 10 - Prob. 1PPCh. 10 - Prob. 2PPCh. 10 - Practice Problem 10.3 How would the molecular ion...Ch. 10 - Prob. 4PPCh. 10 - Prob. 5PPCh. 10 - Prob. 6PPCh. 10 - Practice Problem 10.7 Chlorination reactions of...Ch. 10 - Prob. 8PPCh. 10 - Prob. 9PPCh. 10 - Prob. 10PP
Ch. 10 - Prob. 11PPCh. 10 - Practice Problem 10.12 Benzylic radicals, due to...Ch. 10 - Prob. 13PPCh. 10 - Practice Problem 10.14 Show how the following...Ch. 10 - Prob. 15PPCh. 10 - Prob. 16PPCh. 10 - Prob. 17PPCh. 10 - Prob. 18PCh. 10 - Explain the relative distribution of produces...Ch. 10 - 10.20 Which of the following compounds can be...Ch. 10 - Prob. 21PCh. 10 - Prob. 22PCh. 10 - Prob. 23PCh. 10 - Prob. 24PCh. 10 - 10.25 List in order of decreasing stability all of...Ch. 10 - Prob. 26PCh. 10 - Prob. 27PCh. 10 - Prob. 28PCh. 10 - Starting with the compound or compounds indicated...Ch. 10 - Prob. 30PCh. 10 - 10.31 Synthesize each of the following compounds...Ch. 10 - Synthesize each of die following compounds by...Ch. 10 - Prob. 33PCh. 10 - Prob. 34PCh. 10 - Prob. 36PCh. 10 - The halogen atom of an alkyl halide can be...Ch. 10 - Prob. 38PCh. 10 - Prob. 39PCh. 10 - Write a mechanism for the following reaction.Ch. 10 - 10.41 Hydrogen peroxide and ferrous sulfate react...Ch. 10 - Prob. 42PCh. 10 - If one were to try to draw the simplest Lewis...Ch. 10 - Prob. 1LGPCh. 10 - 2. (a) Propose a synthesis of 2-methoxypropene...
Additional Science Textbook Solutions
Find more solutions based on key concepts
The skeleton equation and the balanced chemical equation for the reaction between iron and chlorine needs to be...
Chemistry: Matter and Change
47. A 250.0-mL buffer solution is 0.250 M in acetic acid and 0.250 M in sodium acetate.
a. What is the initial ...
Chemistry: A Molecular Approach
4.1 Write the symbols for the following elements.
a. copper
b. platinum
c. calcium
d. manganese
e. Iron
...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
4. 38 Strontium has four naturally occurring isotopes, with mass numbers 84, 86, 87, arid 88.
a. Write the atom...
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
a. How does increasing the temperature increase the rate of a chemical reaction? b. How does increasing the amo...
General, Organic, and Biological Chemistry (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the steps that are critical in the following reaction in order to explain the stereo-chemical outcome at the carbon marked with the asterisk.arrow_forwardRank the indicated hydrogen atoms in order of increasing ease of abstraction in a radical halogenation reaction.arrow_forwardExplain why free-radical halogenation usually gives mixture of products.arrow_forward
- Give the complete mechanism, including transition states, for the following reaction. Then draw the potential energy diagram for it and for similar alkylbromides of other substitution patterns. Give the rate law for this type of mechanism. Then give the rate trend based on substitution pattern and explain it using the rate law and potential energy diagram you have provided. Use additional sheets, make sure you draw large enough for your work to be clearly understood, and attach them in order. H" -CI HSCH3 ethanol H3CS- + H" -SCH 3 Explain why 1-chlorobicyclo[2.2.1]heptane (shown below) even though it is a tertiary alkyl halide, is virtually unreactive in the SN1 reaction. It has been estimated that it is 10-13 times as reactive as tert-butyl chloride. Hint: consider the preferred geometry of the reactive intermediate.arrow_forwardFor the reaction in part "b", please explain which products are the major and minor products. Also explain which are the kinetic and thermodynamic products.arrow_forwardPlease show the entire mechanism with curved arrows for the reaction. Why is the reaction considered unfavorable in reference to the stability ladder? Why does the reaction favor the product formation? *please show the resonance structures in the reaction.*arrow_forward
- As we will learn, many antioxidants–compounds that prevent unwanted radical oxidation reactions from occurring–are phenols, compounds that contain an OH group bonded directly to a benzene ring.a.) Explain why homolysis of the O–H bond in phenol requiresconsiderably less energy than homolysis of the O–H bond in ethanol(362 kJ/mol vs. 438 kJ/mol).b.) Why is the C–O bond in phenol shorter than the C–O bond in ethanol?arrow_forwardWhat are several products for the following reaction with mechanisms included?arrow_forwardb) Explain in detail what characteristics of the alkyl halide influence whether a mechanism will be SN1 or SN2. c) Explain in detail what characteristics of a nucleophile influence whether a reaction will be SN1 or SN2.arrow_forward
- Write a reaction mechanism and give the final product for the following reaction.arrow_forwardAccording to Hammond's postulate, which of the following is correct? The structure of the transition state of an endothermic reaction will be more similar to the structure of the reagents than to that of the products. The structure of the intermediary in an endothermic reaction will be more similar to the structure of the reagents than to that of the products. The transition state structure of an exothermic reaction will be more similar to reagents than to products. All transition states are more similar to products than reagents All transition states are more similar to reagents than products.arrow_forwardSelect the keyword or phrase that will best complete each sentence. Key terms: backside carbocation elimination frontside hyperconjugation inversion maintenance nucleophile product racemization stronger substitution weaker Alkyl halides undergo A orbital. reactions with Brønsted-Lowry bases. is a sp² hybridized and trigonal planar and contains a vacant p All SN2 reactions proceed with in attack of the nucleophile, resulting of configuration at a stereognic center. Spreading out charge by the overlap of an empty p orbital with an adjacent o bond is called Equilibrium favors the products of nucleophilic substitution when the leaving group is a base than the nucleophile. According the to Hammond postulate, the stability of the determines the rate of its formation. The formation of equal amounts of two enantiomeric products from a single starting material is called A is an electron-rich compound, which donates a pair of electrons to an electron deficient compound, forming a covalent bond.…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License