
Pearson eText for Biochemistry: Concepts and Connections -- Instant Access (Pearson+)
2nd Edition
ISBN: 9780137533114
Author: Dean Appling, Spencer Anthony-Cahill
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 10, Problem 20P
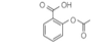
The transport of aspirin (pKa = 3.5, structure shown here) form the digestive tract to the circulation occurs by nonmediated absorption into cells lining the stomach (where pH=0.8) and the small intestine (where pH=6.0). Do you expect absorption to be faster in the stomach or in the small intestine?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The acidity of the stomach is maintained by the H*/K* ATPase in parietal cells of the gastric mucosa.
These cells have an internal pH = 7.4. The H*/K* ATPase transports H* across the cellular membrane into the
stomach where pH = 0.8.
(a) Given a membrane potential of 65 mV (inside negative), calculate AG in kJ/mol for the transport of H* into the
stomach at 37 °C. Show your work.
(b) The hydrolysis of ATP is directly coupled to H* transport and the stoichiometry of the transport reaction is such
that 1 mol of ATP is hydrolyzed for every 1 mol of H* transported into the stomach. ATP is hydrolyzed by the
ATPase on the inside of the cell. Under the conditions given in part (a) and assuming the steady-state
concentrations of ATP, ADP and P₁ given below, is the transport of H* in to the stomach thermodynamically
favorable at 37 °C? Show the calculation that supports your answer.
Steady-state intracellular concentrations:
ATP = 4.2 mM
ADP = 360 μM
Pi = 5.4 mM
For ATP hydrolysis at 37 °C: ATP…
The phosphate buffer (H2PO4 HPO42+ H") in esophageal cells has a pKa of 7.2. If acid floods into
cells, and the pH drops from 7.2 to 3.2, which concentration in the cells will increase, H2PO4 or HPO42?
Is pH 3.2 in the buffer range of this phosphate buffer (pKa 7.2)?
Esophagus
Stomach
HPO42, no
HPO4: yes
H,PO4: yes
H,PO41; no
Polymer beads (resin) made of DEAE (diethylaminoethyl) cellulose are packed in an ion exchange column. The
total mass of beads in the column is 8.47 kg. On average, each bead weighs 0.0023 g and has an average of 18.4
* 10° positively charged amine groups that can adsorba negatively charged protein that passes through the
column. A solution containing 2.07 mg/L of a protein is maintained at pH 6.3 and is passed through the ion
exchange column at 0.215 L/min. The protein has a molecular weight of 154,000. The pk, of the amino groups
on DEAE cellulose is 7.1, and the pl of the protein is 5.6.
2.
A. How long can the column be operated before reaching 80% capacity (i.e., 80% of the amino groups on DEAE
are bound to the protein through an ionic bond)? You may assume that one protein attaches to one +
charge on the beads (although it's possible that proteins attach to more than one + charge).
B. After reaching 80% capacity, explain what you would do to release the protein attached to the…
Chapter 10 Solutions
Pearson eText for Biochemistry: Concepts and Connections -- Instant Access (Pearson+)
Ch. 10 - Prob. 1PCh. 10 - Given these molecular components--glycerol, fatty...Ch. 10 - The classic demonstration that cell plasma...Ch. 10 - The lipid portion of a typical bilayers is about...Ch. 10 - In the following situations, what is the free...Ch. 10 - Propose an experiment that would distinguish...Ch. 10 - Prob. 7PCh. 10 - Peptide hormones (such as insulin) must bind to...Ch. 10 - Prob. 9PCh. 10 - Prob. 10P
Ch. 10 - Prob. 11PCh. 10 - Prob. 12PCh. 10 - Prob. 13PCh. 10 - Prob. 14PCh. 10 - The concentration of glucose in your circulatory...Ch. 10 - ATP is synthesized from ADP, Pi , and a proton on...Ch. 10 - The Na+/ glucose symport transports glucose from...Ch. 10 - Prob. 18PCh. 10 - Prob. 19PCh. 10 - The transport of aspirin (pKa = 3.5, structure...Ch. 10 - Prob. 21P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- (E) Consider an amino acid that has one -amino group (pKa = 9.2), one -carboxyl group (pKa = 1.7) and one ionizable side chain (pKa = 6.2). At which pH range/s would this amino acid be effective as a buffer? If the amino acid shows a net charge of -1 at pH 11 and a net charge of +1 at pH 4, what pKa values would you use to calculate the isoelectric point? At what pH would the net charge be equal to zero?arrow_forward2. 0.1 mL of a protein solution of concentration of 7 mg/mL was diluted to a total volume of 4.0 mL with water (i.e. 0.1 mL of the solution was added to 3.9 mL of water). 3 mL of this solution was then mixed with 27 mL of water. What is the concentration of the diluted protein solution? Space to show your workings:arrow_forwardIf several like-charged proteins are bound simultaneously to an ion exchange column, they can be separated by gradually increasing the salt concentration (applying a “gradient”). A CM-Sepharose column has three proteins bound to it: A (pI = 7.9), B (pI = 7.4), and C (pI = 8.7). At pH = 7.0, the salt concentration on the column is gradually raised from 0 to 500 mM. In what order will the proteins elute? Explain.arrow_forward
- In a TPN bag contains: Kcal of 1000 ml of D50 W, 1000 ml of 7% amino acids, and 650 ml of 20% lipids. A. How many total kcal is provided by this bag of TPN?arrow_forwardThe parietal cells of the stomach lining contain membrane “pumps” that transport hydrogen ions from the cytosol (pH 7.0) into the stomach,contributing to the acidity of gastric juice (pH 1.0). Calculate the free energy required to transport 1 mol of hydrogen ions through these pumps. Assume a temperature of 37 °C.arrow_forwardA mixture of Alanine (pl 6.02), Glutamic Acid (pl 3.22), Glycine (pl 5.79), Lysine (pl 9.74) and Threonine (pl 6.53) are separated by cation exchange chromatography. What is the order of elution of these amino acids if you use gradient buffer system from pH 10 to pH 2 ? v First 1. Alanine v Second 2. Glutamic Acid v Third 3. Glycine v Fourth 4. Lysine v Fifth 5. Threonine 6. No separationarrow_forward
- A tetrapeptide, glutamate-glycine-alanine-lysine, is prepared at at concentration of 1 mM (0.001 M) and is measured in the standard setup (pathlength of 1 cm). What is the approximate absorbance of this peptide at 280 nm? Hint: if the peptide contained a single tryptophan, the answer would be about 10. 10 280 1 0arrow_forwardWe want to measure the activity of alanine aminotransferase (ALAT) present in a serum. The reaction catalyzed by the enzyme is: Reaction 1: +H3N- glutamate H C CH₂ CH₂ COO -COO + pyruvate CH3 C=0 0.1 M phosphate buffer pH 7.4 : 550 µL 1.2 M alanine : 100 μL CH3 time (min) A340 COO COO™ pyruvate lactate dehydrogenase* (LDH, 300 µg.mL-¹): 50 μL 1.5 mM NADH: 200 μL 0.04 M a-ketoglutarate: 500 μL serum containing ALAT: 600 μL The enzyme reaction is realized in the following conditions: In a 1 cm-cuvette are added: 0 0.915 ALAT NADH + H+ LDH a-cétoglutarate COO * Lactate dehydrogenase (LDH) reduces pyruvate into lactate, with the concomitant oxydation of NADH. This allows to indirectly measure the amount of product formed. Reaction 2: NAD+ 1 0.741 C=O H CH₂ CH₂ COO™ CH3 C-OH COO lactate The reaction is performed at 25 °C and the absorbance at 340 nm is monitored every minute, for 5 min. The absorbance values are given in the table below: Data: alanine ENADH at 340 nm = 6220 M¹.cm1. One…arrow_forwardPredict the direction of migration for the following amino acids in solutions of the specified pH. Write Isoelectric, if no migration occurs: Lys at pH=12 (pk values: 2.2; 9.2; 10.8) Glu at pH= 2.0 (pk values: 2.1; 9.5; 4.1) Leu at pH= 6.0 (pk vaues: 2.3; 9.7)arrow_forward
- Protein concentration can readily be determined using the Beer-Lambert law: A = e l c where A = absorbance e = molar absorption coefficient (M-1cm-1) l = light path length (cm) c = concentration (M) If the molar absorption coefficient at 280 nm for yeast ADH is 48860 M-1cm-1 and a 10 mL solution of the protein has an absorbance at 280 nm of 0.4 (as measured by a spectrometer with pathlength 1 cm), then what is the concentration of the protein solution (in μM)? i.e. concentration = ______ μM If the molecular weight of the protein is 36849, what is its concentration in mg/mL? i.e. concentration = _______ mg/mL For each part of the question, show your calculations to arrive at your answers.arrow_forwardWhat is the molecular weight of the Botulinum neurotoxin, a protein that contains 1350 amino acids? Assume an average distribution of amino acidsarrow_forwardA mixture of Alanine (pl 6.02), Glutamic Acid (pl 3.22), Glycine (pl 5.79), Lysine (pl 9.74) and Threonine (pl 6.53) are separated by anion exchange chromatography. What is the order of elution of these amino acids if you use gradient buffer system from pH 10 to pH 2 ? v First 1. Alanine v Second 2. Glutamic Acid v Third 3. Glycine 4. Lysine v Fourth 5. Threonine v Fifth 6. No separationarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license