
(a)
Interpretation:
The stereo isomeric product should be given when the
Concept introduction:
An alkene undergoes an oxidation reaction with Osmium tetra oxide and followed by hydrolysis to give a cis 1, 2
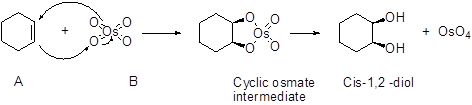
(b)
Interpretation:
The stereo isomeric product should be given when the alkenes reaction with osmium tetroxide followed by hydrogen peroxide hydrolysis.
Concept introduction:
An alkene undergoes an oxidation reaction with Osmium tetra oxide and followed by hydrolysis to give a cis 1, 2 diol for example,
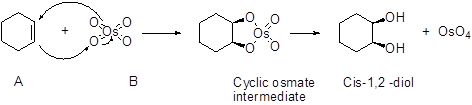
(c)
Interpretation:
The stereo isomeric product should be given when the alkenes reaction with osmium tetroxide followed by hydrogen peroxide hydrolysis.
Concept introduction:
An alkene undergoes an oxidation reaction with Osmium tetra oxide and followed by hydrolysis to give a cis 1, 2 diol for example,
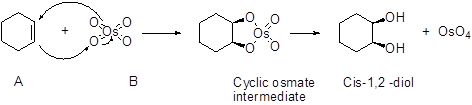
(d)
Interpretation:
The stereo isomeric product should be given when the alkenes reaction with osmium tetroxide followed by hydrogen peroxide hydrolysis.
Concept introduction:
An alkene undergoes an oxidation reaction with Osmium tetra oxide and followed by hydrolysis to give a cis 1, 2 diol for example,
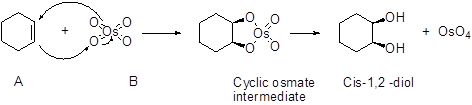
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Pearson eText Organic Chemistry -- Instant Access (Pearson+)
- 1. What alkene will produce the HIGHEST yield of the alkyl halide below? 2. Which of the following alkynes can be deprotonated by NaNH2 in liquid NH3? a. Pent-2-yne b. Hex-3-yne c. none of these d. 3-Methylbutyne e. 3-Methylhex-2-ynearrow_forwardWhat is the major product of m-nitrotoluene chlorination?arrow_forward14. What is the major organic product obtained from the following reaction? H₂ H₂C-C=C-CH₂ a. butane b. 1-butene c. cis-2-butene d. trans-2-buteng Lindlar catalyst inarrow_forward
- Cyclohexene (an alkene) is mixed with potassium permanagnate solution (KMnO4). What do you expect to observe? A. The mixture will turn purple as the permanganate is reduced by the alkene. B. The purple colour will disappear as the permanganate is reduced by the alkene. C. Nothing. Permanganate does not react with alkenes. D. The purple colour will disappear as the permanganate is oxidised by the alkene.arrow_forward1. Which reaction conditions would be best for turning ethene into ethanol? a.H2O, HBr b. CH3OH, H2SO4 c.CH3OH, HBR d. H2O, H2SO4 2. Which alkene would be non-regioselective in a reaction with HBr? a. 2-ehyl-2-methyl-2-butene b.2-ethyl-3-methyl-2-butene c. 2,3-dimethyl-2-butene d.1,2-dimethyl-2-butenearrow_forwardSelect the best reagent expected to convert 3-heptyne to cis-3-heptene. A. NaNH2, NH3 B. Na, NH3 C. H2, Lindlar’s catalyst D. Both A and C E. Both B and Carrow_forward
- Which of the following alkyl halides will not react to either NaI in acetone and 2% ethanolic AgNO3? a. 2-Methyl-2-Chloropropane b. Bromobenzene c. 1-Chloropentane d. 1-Chlorocyclopentanearrow_forwardWhen free radical halogenation is performed with one of the compounds below, only a single product with the formula C5H11Cl is isolated. Which compound fits this description? Select one: a. 2,2-dimethylpropane b. cyclopentane c. pentane d. 2-methylbutanearrow_forwardD10)arrow_forward
- 57. The order of decreasing ease of dehydrohalogenation OCH₂C I. >-CH2CH2CI A. I>II>III>IV B. CH3CH₂CHCICH3 C. II. CHOHCH₂CH3 D. CH3CHBRCH₂CH3 CH₂CH2C1 B. III>II>I>IV 58. Which of the following reactions will produce the highest percentage yield of 1-butene. OH A. CH3CH₂CH(N* (CH3)3)CH3 alc. KOH conc. H₂SO4 heat NaOC₂H5 III. C₂H5OH of the following compounds is: CH2CH₂CI CH₂CH₂C1 CH₂ -CH₂CH2CI C. III>IV>II> I IV. D. IV>III>II>Iarrow_forwardMetathesis of which of the following sets of alkenes leads to the highest yield of a single alkene? 1. 1-butene and 1-pentene 2. 2-butene and 3-hexene 3. 2-butene and 1-pentene b. What kinds of alkenes should be used in metathesis reactions that use two different alkenes as starting materialsarrow_forwardWhich of the following concepts explains Markovnikov's rule as applied to the addition of HBr to propene?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

