
Concept explainers
(a)
Interpretation:
The reaction and complete, detailed mechanism for the reaction is to be drawn.
Concept introduction:
A strong Bronsted acid such as
Answer to Problem 11.27P
The overall reaction for the given compound is
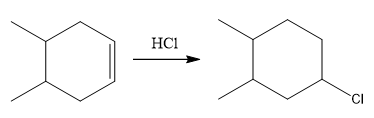
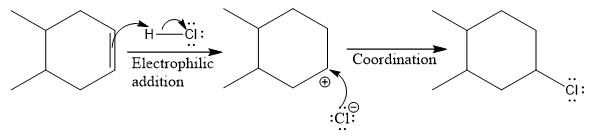
The complete mechanism for the above reaction is
Explanation of Solution
The structure for the given compound
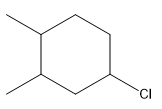
The compound shown above is the alkyl halide compound. This compound is formed by an electrophilic addition reaction of alkene with a strong Bronsted acid
The overall reaction for the given compound is

The above reaction completes in two steps. In the first step, the electrophilic addition step, the proton is added to one carbon with C=C bond, forming the carbocation intermediate. The second step is the coordination step in which the nucleophile attacks the carbocation to yield the product.
The complete mechanism for the above reaction is

The reaction and complete, detailed mechanism for the reaction is drawn on the basis of the mechanism of electrophilic addition reaction of alkenes with strong acid.
(b)
Interpretation:
The reaction and complete, detailed mechanism for the reaction is to be drawn.
Concept introduction:
Alkyl halide is the product of the electrophilic addition reaction of the alkene with a strong Bronsted acid such as
A strong Bronsted acid such as
Answer to Problem 11.27P
The overall reaction for the given compound is
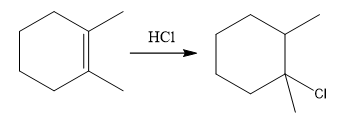
The complete mechanism for the above reaction is
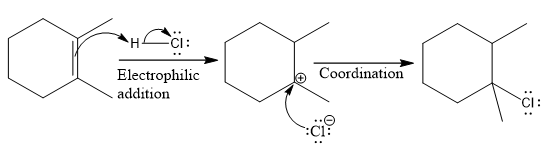
Explanation of Solution
The structure for the given compound
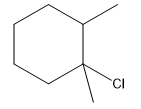
The compound shown above is the alkyl halide compound. This compound is formed by an electrophilic addition reaction of an alkene with a strong Bronsted acid
The overall reaction for the given compound is

The above reaction completes in two steps. In the first step, the electrophilic addition step, the proton is added to one carbon with C=C bond, forming the carbocation intermediate. The second step is the coordination step in which the nucleophile attacks the carbocation to yield the product.
The complete mechanism for the above reaction is

The reaction and complete, detailed mechanism for the reaction is drawn on the basis of the mechanism of electrophilic addition reaction of alkenes with strong acid.
(c)
Interpretation:
The reaction and complete, detailed mechanism for the reaction is to be drawn.
Concept introduction:
Alkyl halide is the product of the electrophilic addition reaction of the alkene with a strong Bronsted acid such as
A strong Bronsted acid such as
Answer to Problem 11.27P
The overall reaction for the given compound is
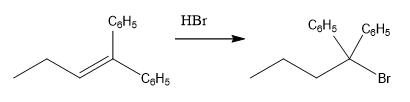
The complete mechanism for the above reaction is
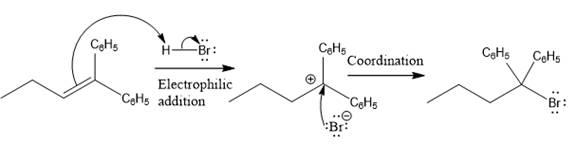
Explanation of Solution
The structure for the given compound
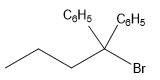
The compound shown above is the alkyl halide compound. This compound is formed by an electrophilic addition reaction of alkene with a strong Bronsted acid
The overall reaction for the given compound is

The above reaction completes in two steps. In the first step, the electrophilic addition step, the proton added to the internal carbon, forming the the more stable carbocation intermediate, which is tertiary and resonance stabilized. The second step is the coordination step in which the nucleophile attacks the carbocation to yield the product.
The complete mechanism for the above reaction is
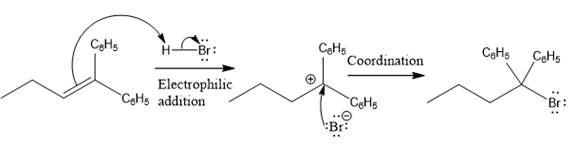
The reaction and complete, detailed mechanism for the reaction is drawn on the basis of the mechanism of electrophilic addition reaction of alkenes with strong acid.
(d)
Interpretation:
The reaction and complete, detailed mechanism for the reaction is to be drawn.
Concept introduction:
Alkyl halide is the product of the electrophilic addition reaction of the alkene with a strong Bronsted acid such as
A strong Bronsted acid such as
Answer to Problem 11.27P
The overall reaction for the given compound is
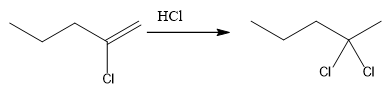
The complete mechanism for the above reaction is
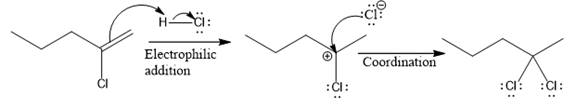
Explanation of Solution
The structure for the given compound
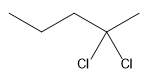
The compound shown above is the alkyl halide compound. This compound is formed by an electrophilic addition reaction of alkene with a strong Bronsted acid
The overall reaction for the given compound is

The above reaction completes in two steps. In the first step, the electrophilic addition step, the proton is added to the internal carbon, forming the the more stable carbocation intermediate, which is tertiary. The second step is the coordination step in which the nucleophile attacks the carbocation to yield the product.
The complete mechanism for the above reaction is
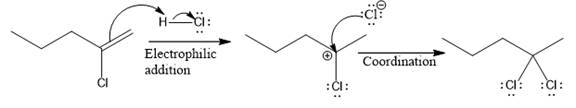
The reaction and complete, detailed mechanism for the reaction is drawn on the basis of the mechanism of electrophilic addition reaction of alkenes with strong acid.
Want to see more full solutions like this?
Chapter 11 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- Draw the complete, detailed mechanism for the reaction shown here. Will the product be optically active? Explain.arrow_forwardHelp, explain in detail please. Thank you! Did the following overall reaction occur by an SN2, SN1, E2, or E1 mechanism? How do you know? Draw the complete, detailed mechanism to account for the formation of both products.arrow_forwardPredict the major product of each of the reactions shown here and provide the complete, detailed mechanism.arrow_forward
- Help, explain in detail please. Thank you! Did the overal reaction shown here occur by and SN2, Sn1, E2, or E1 mechanism? How do you know? Draw the complete, detailed mechanism to account for the formation of both products.arrow_forwardCan you please help with the following organic chemistry reaction (see attached image) Provide the mechanism involved in the reaction and what the major product(s) would be. Thank youarrow_forwardNoting the curved arrows, draw all the product(s), organic and inorganic, of the following reaction.arrow_forward
- Determine the major product of each reaction in Problem and draw the complete, detailed mechanism. Pay attention to stereochemistry where appropriate.arrow_forwardProvide the full mechanism and major products for each of the following reactions.arrow_forwardprovide the major product(s) for each of the following reactions. also, provide the mechanism for the last two reactionsarrow_forward
- Draw the complete mechanism and the major organic product for each of the following reactions.arrow_forwardThe reaction shown here is a halosulfonation, which is a useful variation of the sulfonation reaction. Draw the complete mechanism for this reaction.arrow_forwardNeed the product, mechanism, pka, conjugates labeled, and direction of equation for: Phenol & triethylaminearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





