
Concept explainers
(a)
Interpretation:
The major product of the given electrophilic addition reaction is to be predicted.
Concept introduction:
Strong Bronsted acids add to the double bond in a two-step electrophilic addition reaction to replace the
Answer to Problem 11.52P
The major product of the given reaction is
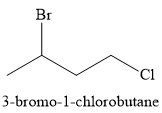
Explanation of Solution
The given reaction is
The substrate is an
In the first step, the
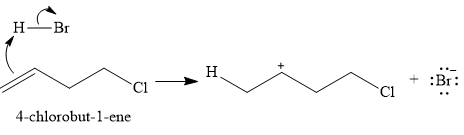
In the second step, the conjugate base
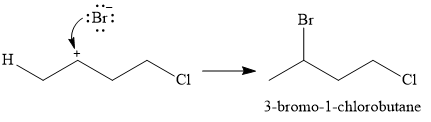
Thus, for the given reaction, the major product formed is

Strong Bronsted acids add to a double bond, replacing the
(b)
Interpretation:
The major product of the given electrophilic addition reaction is to be predicted.
Concept introduction:
In a two-step electrophilic addition reaction, the strong Bronsted acids add to the double bond to replace the
Answer to Problem 11.52P
For the given reaction, the major product formed is,
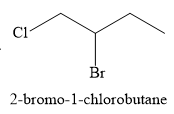
Explanation of Solution
The given electrophilic addition reaction is
The first step of the reaction is electrophilic addition of the proton from the acid. Two carbocations are possible when the
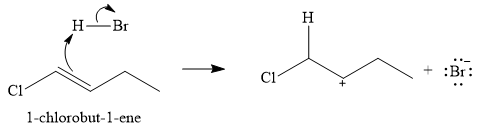
The conjugate base
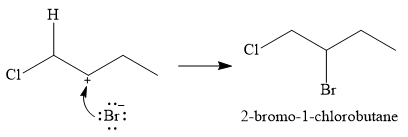
Thus, for this reaction, the major product formed is,

Strong Bronsted acids add to a double bond, replacing the
(c)
Interpretation:
The major product of the given electrophilic addition reaction is to be predicted.
Concept introduction:
In a two-step electrophilic addition reaction, the strong Bronsted acids add to the double bond to replace the
Answer to Problem 11.52P
For this reaction, the major product formed is,
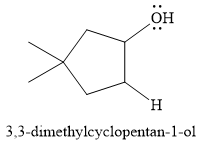
Explanation of Solution
The given reaction is
The overall reaction is one of hydration of the double bond, i.e., the addition of water. Water is a weak acid, but in the presence of a strong acid (denoted by the
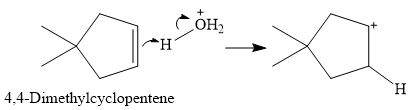
In the next step, the conjugate base
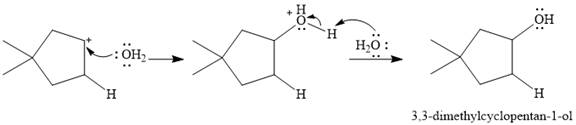
Thus, for this reaction, the major product formed is,

Strong Bronsted acids add to a double bond, replacing the
(d)
Interpretation:
The major product of the given electrophilic addition reaction is to be predicted.
Concept introduction:
In a two-step electrophilic addition reaction, the strong Bronsted acids add to the double bond to replace the
Answer to Problem 11.52P
For the given reaction, the major product formed is,
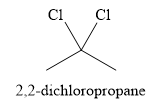
Explanation of Solution
The given reaction is
There are two
In the first step, an alkenyl carbocation is formed by addition of proton the triple bond. A more stable carbocation, with the positive charge on the secondary carbon, is produced.

In the second step, the conjugate base
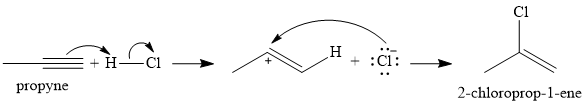
Since the product has a
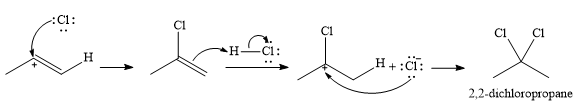
Thus, for this reaction, the major product formed is,

Strong Bronsted acids add twice to a triple bond, replacing each
(e)
Interpretation:
The major product of the given electrophilic addition reaction is to be predicted.
Concept introduction:
In a two-step electrophilic addition reaction, the strong Bronsted acids add to the double bond to replace the
Answer to Problem 11.52P
For the given reaction, the major product formed is,
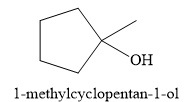
Explanation of Solution
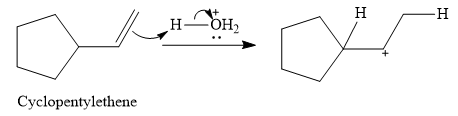
The given electrophilic addition reaction is
![]()
The acid in this case is
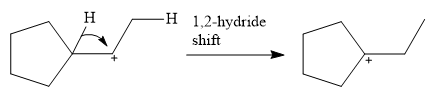
This carbocation is prone to rearrangement. A
The conjugate base
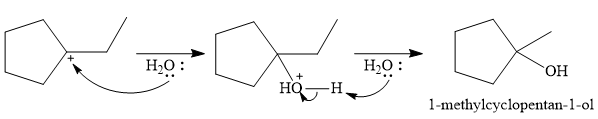
Thus, for the given reaction, the major product formed is,

Strong Bronsted acids add to a double bond, replacing the
Want to see more full solutions like this?
Chapter 11 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
