Given that volume of base,
NaOH
and it is denoted by
Vb.
Given the strength (
M1
) and volume of Malonic acid
HO2CCH2CO2H
solution (
V1
) and strength of
KOH
(
M2
), Since malonic acid is a diprotic acid two equivalence points do occur.
The reaction occurring in titration of malonic acid with
NaOH
is represented as,
HO2CHCH2CO2H+OH−→O2−CHCH2CO2H+H2OO2−CHCH2CO2H + OH− →O2−CHCH2CO2−
The volume of
NaOH
added to reach first equivalence point (
V1
)is calculated as,
V1M1 = V2M250.0 mL× 0.0500 M = V2×0.100MV2 = 50 mL× 0.0500 M0.100M = 25.0 mL
Thus at first equivalence point
50mL
of acid is converted to
50mL
of monobasic anion. Upon continual addition of base another proton left in monobasic anion is abstracted and dibasic anion is formed. Thus at second equivalence point,
50mL
of monobasic anion is converted to
50mL
of dibasic anion.
The volume of
NaOH
added to reach second equivalence point (
V2
)is calculated as,
V1M1 = V2M250.0 mL× 0.0500 M = V2×0.100MV2 = 50 mL× 0.0500 M0.100M = 25.0 mL
Thus after the addition of
25.0mL
of base we further need to add
25.0mL
of base to reach the second equivalence point. Hence totally
25.0mL+25.0mL=50.0mL
of base is required to reach second equivalence point from the beginning of the titration.
Let
H2M
be malonic acid. When volume of base added is
0.00mL
, that is in absence of base, the
pH
depends upon the dissociation of malonic acid. Since it is a weak acid it doesn’t dissociate completely into individual ions. The acid has two Ka values – K1 and K2 since it is diprotic acid.
H2M⇌H++HM−
[H+]=[HM−]=x, [H2M]=0.0500−x
and
K1 of H2M is 1.42×10−3
Ka
for the above reaction is,
Ka = x20.0500−x1.42×10−3 = x20.0500−x
Solving for ‘x’,
x=7.75×10−3M=[H+]
pH
is determined as,
pH = - log[H+] = -log(7.75×10−3) = 2.11
When volume of
NaOH
added is
8.0mL
, the base begins to react with acid. Upon reaction with base the acid forms conjugate base. The titrating mixture then becomes buffer. The concentration of conjugate base formed increases with addition of base at each step. Using Henderson-Hasselbalch equation
pH
can be determined.
The titration reaction at this instant is,
H2M+OH−→HM−+H2O
Their respective concentration is,
H2M + OH− → HM− + H2O_______________________________________________________Initial: 25 mL 8 mL - -Final: 25-8 mL - 8 mL -
Substitute the values known to determine
pH
,
pH = pK1+ log[HM−][H2M] = 2.847 +log(817) = 2.52
When volume of
NaOH
added is
12.5mL
, the base begins to react with acid. Upon reaction with base the acid forms conjugate base. The titrating mixture then becomes buffer. The concentration of conjugate base formed increases with addition of base at each step. Using Henderson-Hasselbalch equation
pH
can be determined.
The titration reaction at this instant is,
H2M+OH−→HM−+H2O
Their respective concentration is,
H2M + OH− → HM− + H2O_______________________________________________________Initial: 25 mL 12.5 mL - -Final: 25-12.5 mL - 12.5 mL -
Substitute the values known to determine
pH
,
pH = pK1+ log[HM−][H2M] = 2.847 +log(12.512.5) = 2.85
When volume of
NaOH
added is
19.3mL
, the base begins to react with acid. Upon reaction with base the acid forms conjugate base. The titrating mixture then becomes buffer. The concentration of conjugate base formed increases with addition of base at each step. Using Henderson-Hasselbalch equation
pH
can be determined.
The titration reaction at this instant is,
H2M+OH−→HM−+H2O
Their respective concentration is,
H2M + OH− → HM− + H2O_______________________________________________________Initial: 25 mL 19.3 mL - -Final: 25-19.3 mL - 19.3 mL -
Substitute the values known to determine
pH
,
pH = pK1+ log[HM−][H2M] = 2.847 +log(19.35.7) = 3.38
When volume of
NaOH
added is
25.0mL
,
25.0mL
of the acid has been converted to its conjugate base, monobasic anion as the first equivalence point is reached.
formal concentration of malonic acid at this instant is,
F = (50mL50mL+25mL)×0.0500M=0.0333M
Hence
[H+]
is determined as,
[H+] = K1+K2F+K1KwK1+F = (1.42×10−3)+(2.01×10−6×0.0333)+(1.42×10−3×1×10−14)(1.42×10−3)+0.0333
Solving the above equation,
[H+]=5.23×10−5M
Substitute the values known to determine
pH
,
pH = -log[H+] = -log(5.23×10−5) = 4.28
After reaching the first equivalence point, continual addition of base forms dibasic anion.
When volume of base added is
37.5mL
,
Volume of base is equivalent to,
Vb=32Ve
Therefore,
pH = pK2+ log[HM−][H2M] = 5.70 +log(37.537.5) = 5.70
When volume of base added is
50mL
, second equivalence point is reached as calculated previously. All of the monobasic anion, HM- is completely converted to dibasic anion, M2-. Hydrolysis of dibasic anion determines the
pH.
The reaction is
M2−+H2O⇌HM−+OH−
Formal concentration of malonic acid is,
(50mL50mL+50mL)×0.0500M=0.025M
Then
[HM−]=[OH−]=x, [M2−]=0.025−x
Kb
of malonic acid is,
Kb = KwKa2 = 10−142.01×10−6
Also,
Kb = x20.0250−x10−142.01×10−6 = x20.0250−x
Solving the above equation for ‘x’,
x=[OH−]=1.12×10−5M
Substitute the values known to determine
pH
,
pH+pOH = 14pH = 14 - pOH = 14-(-log [OH−]) = 14-(-log(1.12×10−5)) = 14 - 4.95 = 9.05
When volume of acid added is
56.3mL
, concentration of
[OH−]
by excess addition of sodium hydroxide determines the
pH.
[OH−] =(56.3−50 mL106.3 mL)×0.100M = 5.93×10−3M
Substitute the values known to determine
pH
,
pH+pOH = 14pH = 14 - pOH = 14-(-log [OH−]) = 14-(-log(5.93×10−3)) = 14 - 2.23 = 11.77
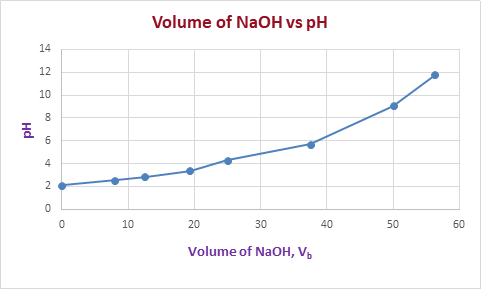
The graph of
pH
versus volume of
NaOH
is plotted as,



 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY




