
Concept explainers
(a)
Interpretation: The alkyl halide that is used to form the given carboxylic acid has to be identified.
Concept introduction: The reaction of
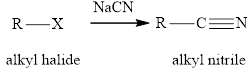
The hydrolysis reaction of alkyl nitrile in presence of acid is written as:
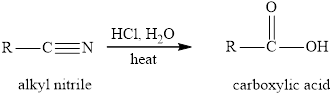
(b)
Interpretation: The alkyl halide that is used to form the given carboxylic acid has to be identified.
Concept introduction: The reaction of alkyl halides with sodium cyanide gives alkyl cyanides or nitriles. After the reaction there is increase incarbon atoms of the alkyl chain of alkyl halide. The nitriles obtained undergo hydrolysis reaction in presence of acid and gives carboxylic acid. The general reaction of alkyl halides and sodum cyanide is written as:
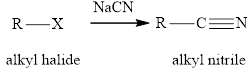
The hydrolysis reaction of alkyl nitrile in presence of acid is written as:
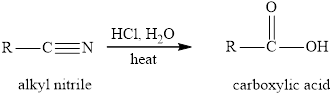
(c)
Interpretation: The alkyl halide that is used to form the given carboxylic acid has to be identified.
Concept introduction: The reaction of alkyl halides with sodium cyanide gives alkyl cyanides or nitriles. After the reaction there is increase incarbon atoms of the alkyl chain of alkyl halide. The nitriles obtained undergo hydrolysis reaction in presence of acid and gives carboxylic acid. The general reaction of alkyl halides and sodum cyanide is written as:
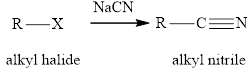
The hydrolysis reaction of alkyl nitrile in presence of acid is written as:
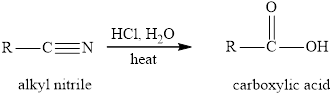
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Essential Organic Chemistry, Global Edition
- Tollen's test is a qualitative laboratory test used to distinguish between aldehydes and ketones. The reagent uses an aqueous solution of _____________. Aliphatic compounds would produce what color in Brady's test Jones Oxidation test uses ________ and sulfuric acid as reagentsarrow_forwardWhat type of alcohol is produced from the reaction of carboxylic acid with Grignard reagent? Explain and draw the product.arrow_forwardWhich will undergo hydrolysis to form a carboxylic acid?arrow_forward
- What the Reaction of Hydrolysis of an Ester with a Primary or Secondary Alkyl Group.arrow_forwardWhat type of product should be formed if butanol went through an oxidation reaction to completion? (A) carboxylic acid (B) ketone (C) no product formed (D) aldehydearrow_forwardWhat carboxylic acid is used to prepare this ester?arrow_forward
- An aldehyde can be oxidized to produce a carboxylic acid. Draw the carboxylic acid that would be produced by the oxidation of butanal.arrow_forwardWhat type of alcohol is produced from the reaction of carboxylic acid with Grignard reagent?arrow_forwardGive one reason why benzene can act as an acid in the following reaction,arrow_forward
- Organolithium is another organometallic used in organic synthesis. Give an example of an organic synthesis that uses organolithium.arrow_forwardWhat is the organic product formed when BzCl reacts with aqueous NaOH. BzCl = benzoyl chloride.arrow_forwardIf an aldehyde is oxidized, what type of compound would be produced? a. an alcohol b. a ketone c. a carboxylic acid d. a different aldehydearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

