
Concept explainers
(a)
Interpretation:
To write the mechanism for an uncatalyzed hydrolysis of methyl propionate.
Concept Introduction:
The hydrolysis reaction of ester is the reaction in which the breaking of ester bond is done by using water molecule in presence of acid or base as catalyst. The product obtained after the ester hydrolysis is the corresponding carboxylic acid and an alcohol. The hydrolysis reaction can be done in the presence of an acid or base to increase the
The general reaction of an ester to produce in presence of only water is written as,
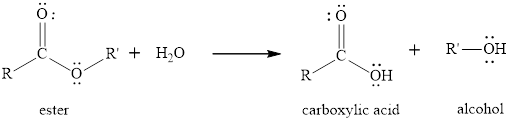
(b)
Interpretation:
To write the mechanism for aminolysis of phenyl formate using methyl amine.
Concept Introduction:
The reaction of a carboxylic acid derivative with ammonia, primary and secondary
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Essential Organic Chemistry, Global Edition
- A fellow student somehow mixed an aspirin sample with paracetamol after synthesising both. Explain how you would separate aspirin from paracetamol using liquid-liquid extraction.arrow_forward1. Why does p-nitroacetanilide recrystallize in ethanol, but the o-acetanilide remains in the filtrate? 2. Give two reasons why a water bath can and must be used during the recrystallisation of benzoin synthesisis.arrow_forwardExplain the mechanism for an acid catalyzed hydrolysis of nitrile ?arrow_forward
- How to use streckers synthesis to prepare phenylalanine in the laboratoryarrow_forwardWhat is the most likely organic product of the exhaustive hydrolysis of PhCN? A. benzoic acid B. benzamide C. benzylamine D. benzenearrow_forwardNinhydrin reacts with an amino acid to form a purple-colored compound. Propose a mechanism to account for the formation of the colored compound.arrow_forward
- What are at least three sources of error for the synthesis of acetanilide lab?arrow_forwardSuggest a test you will use to show that a given food substance contains protein. Show how you will use; A modified Gabriel's synthesis A Streckers' synthesis to prepare phenylalanine in the laboratory.arrow_forwardWhy is retrosynthesis seen as a very important approach in the synthesis of organic compounds, explain the steps in retrosynthesis and apply them in the synthesis of p-amino benzoate analgesics. Why is a protective group needed in the synthesis stage ?arrow_forward
- Given the information pictured, The final step of the reaction sequence uses PhLi. Provide a reason and possible mechanism by which this final elimination and hydrolysis reaction would occur.arrow_forwardPropose a mechanism for the acid-catalyzed reaction of salicylic acid with aceticanhydride.(b) Explain why a single drop of sulfuric acid dramatically increases the reaction ratearrow_forwardSynthesis of p-Bromoaniline Why is a sodium acetate solution added in part 1? a) The sodium ion directs the position of the acetyl group. b) To protonate the intermediate and make it less soluble. c) The acetate catalyzes the formation of the amide bond. d) To deprotonate the intermediate and make it less solublearrow_forward

 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

