
Interpretation:
The major
Concept introduction:
Elimination reaction:
An elimination reaction is removal of two substituents in a molecule and forms alkene. An elimination reaction is one or two-step process which based on the mechanism when two substituents removed from the molecule in single step is called E2 reaction. When two substituents are removed from the molecule in two steps is called E1 reaction.
E1 elimination:
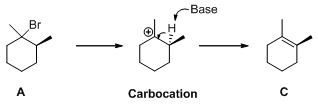
Saytzeff's Rule:
It is elimination reaction in which the formation of olefin on most hindered or highly substituted position.
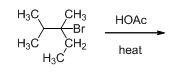
Given information:
The given compound is shown below,
Trending nowThis is a popular solution!

Chapter 11 Solutions
Bundle: Organic Chemistry, Loose-leaf Version, 9th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card
- 3H. Put it all together. Predict the E1 products of the following reaction. Label alkenes as E/Z. 4. Predict the E1 products of the following reaction. Label akenes as E/Z.arrow_forwardGive the major and minor products of the following E1 reaction and identify each and name each productarrow_forwardPredict the major product of E1 elimination of the following compounds and write a detailed mechanism for its formationarrow_forward
- Predict the elimination products of the following reactions, and label the major products. trans-1-bromo-2-methylcyclohexane + NaOCH3 in CH3OHarrow_forwardPredict the major products of the following reactions.(a) (R)-2-hexyl tosylate + NaCN(b) the tosylate of cyclohexylmethanol + excess NH3arrow_forwardWhat is the mechanism to reach the major product of the reaction below?arrow_forward
- Predict the products of the following reactions.(a) cyclohexylmethanol + TsCl/pyridine (b) product of (a) + LiAlH4arrow_forwardPredict the major products of the following reactions. p@xylene + Na (liquid NH3, CH3CH2OH)arrow_forwardI. Propose a mechanism reaction for the reaction below. 2. Identify the major and the minor products.arrow_forward
