
Concept explainers
The concentration of induced Na and Cl radioactivity in the cooling water after a single passage through the reactor core if the mean thermal flux is 1011neutrons per cm2/s and the mean temperature in the core is 80o C.
Answer to Problem 12.1P
Explanation of Solution
Given info:
Water boiler reactor core at a rate is
Coiled stainless steel tube’s inside diameter is
Coiled stainless steel tube’s length is
The concentration of Na and Cl in the water is 5atoms each per million molecules
Mean thermal flux is
The mean temperature in the core is
Formula used:
Activity,
Distance
The number of radioactive atoms/L produced by thermal neutron irradiation of N target atoms/L during an irradiation time t seconds,
Calculation:
Since the irradiation time of one passage through the core is much less than the
The half-life of the activated isotopes, the resulting activity is:
To correct for the cross-section at a different temperature, the following equation is applied to the cross-sections (Etherington):
The following reactions and their associated parameters are used for this problem.
Also listed is the number of target atoms per liter for each element, which were calculated as shown below.
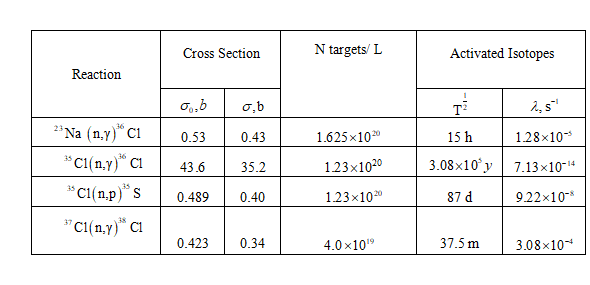
Calculate the number of atoms of each nuclide present per
Since there are 5 atoms Na and Cl for every million molecules of water;
There are two isotopes of Cl:
and
Calculate the irradiation time that is, the time that the water spends in the reactor
For the case of
And, the activity is
Activity
Activity
By similar calculations, we find that for the other activations:
Conclusion:
The concentration of induced
Want to see more full solutions like this?
- What is the dose in mSv for: (a) a 0.1 Gy xray? (b) 2.5 mGy of neutron exposure to the eye? (c) 1.5 mGy of exposure?arrow_forwardData from the appendices and the periodic table may be needed for these problems. Show that the activity of the 14C in 1.00 g of 12C found in living tissue is 0.250 Bq.arrow_forwardFind the radiation dose in Gy for: (a) A 10-mSv fluoroscopic X-ray series, (b) 50 mSv of skin exposure by an a emitter, (c) 160 mSv of and rays from the 40K in your body.arrow_forward
- (a) How many 239Pu nuclei must fission to produce a 20.0kT yield, assuming 200 MeV per fission? (b) What is the mass of this much 239Pu?arrow_forwardCalculate the dose in Sv to the chest at a patient given an xray under the following conditions. The xray beam intensity is 1.50 W/m2, the area of the chest exposed is 0.0750 m2 35.0% of the xrays are absorbed in 20.0 kg of tissue, and the exposure time is 0.250 s.arrow_forwardData from the appendices and the periodic table may be needed for these problems. A 60Co source is labeled 4.00 mCi, but its present activity is found to be 1.85107Bq. (a) What is the present activity in mCi? (b) How long ago did it actually have a 4.00—mCi activity?arrow_forward
 Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax




