
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
9th Edition
ISBN: 9781292151229
Author: Wade, LeRoy G.
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.23SP
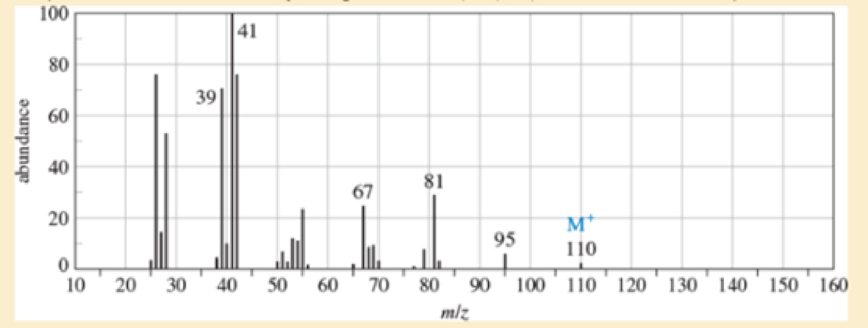
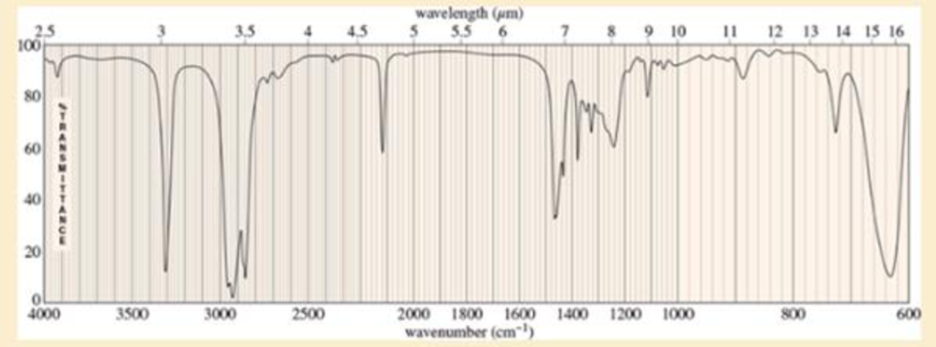
An unknown, foul-smelling hydrocarbon gives the mass spectrum and infrared spectrum shown.
- a. Use the mass spectrum to propose a molecular formula. How many elements of unsaturation are there?
- b. Use the IR spectrum to determine the functional group(s), if any.
- c. Propose one or more structures for this compound. What parts of the structure are uncertain? If you knew that hydrogenation of the compound gives n-octane, would the structure still be uncertain?
- d. Propose structures for the major fragments at 39, 67, 81, and 95 in the mass spectrum.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
You may draw the structures in either Kekule, condensed, or mixed format initially. In the tables draw them as skeletal structures.
Attach the sheet of paper with your DEPT spectra at the end of this packet before turning your packet in.
answer with explanation
Step 5: Draw the structure that best fits the mass spec data.
Let's review the results of the previous steps.
Step 1: The molecular ion is 27.
Step 2: There are no Cl or Br atoms based on isotope pattern.
Step 3: The molecular ion is odd, so there is one nitrogen.
Now consider Step 4 as it relates to the unknown compound.
Step 4: examine the largest peaks and calculate the difference from the molecular ion. Look for common alkyl fragments.
The mass spec data shows only one base peak at m/z 27, and a smaller peak at 26, so there are no alkyl fragments. One nitrogen
atom will have a molecular weight of 14, leaving 13 amu for the remaining unknown portion. A molecular weight of 13 amu
can only correspond to one carbon atom and one nitrogen atom, giving the molecular formula of CHN.
Deduce the structure of the unknown compound.
Select
Draw
Rings
More
Erase
H
Chapter 12 Solutions
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Real walls are never totally adiabatic. Use your experience to order the following walls in increasing order wi...
Thermodynamics, Statistical Thermodynamics, & Kinetics
Practice Problem 1.22 Which of the following alkenes can exist as cis-trans isomers? Write their structures. Bu...
Organic Chemistry
Problem 11.1 Neopheliosyne B is a novel acetylenic fatty acid isolated from a New Caledonian marine sponge. (a)...
Organic Chemistry
2. Why shouldn’t you work in a laboratory by yourself?
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A) Using the mass spectrum and the IR spectrum, identify any heteroatoms in the compound. Explain your reasoning in a sentence or two. B) Using the mass spectrum, deduce the molecular formula for the compound. Show your calculations. C) Using the IR spectrum, identify all significant IR peaks between 4000 and 1500 cm^-1 . List these peaks and beside each one write the type of bond that gave rise to the peak. D) Propose several structures for compound 1 consistent with your answers to part A-C.arrow_forwardA prominent (M+ . -18) peak suggests that the compound might be what? A. Alkane B. Alcohol C. Ether D. Ketonearrow_forwardLook at the structure of ethyl acetate in your notebook. In which region of the 13C spectrum of ethyl acetate would you NOT expect any peaks? a.0 - 50 ppm b.50 - 100 ppm c.100 - 160 ppm d.160 - 220 ppmarrow_forward
- True or False 1. A molecule that is "IR inactive" means that it does not produce any signal due to no vibration. 2. Infrared spectroscopic data is reported in wavenumber (cm-1) against absorbance because they have a linear relationship. 3. The signals observed from a molecule of chloropropane will have a higher wavenumber than iodopropane. 4. The signals observed from the C-C bond in an alkene will report at a higher wavenumber than the C-C bond in an alkyne.arrow_forwardn, foul-smelling hydrocarbon gives the mass spectrum and infrared spectrum shown.(a) Use the mass spectrum to propose a molecular formula. How many elements of unsaturation are there?(b) Use the IR spectrum to determine the functional group(s), if any.(c) Propose one or more structures for this compound. What parts of the structure are uncertain? If you knew thathydrogenation of the compound gives n-octane, would the structure still be uncertain?(d) Propose structures for the major fragments at 39, 67, 81, and 95 in the mass spectrum.arrow_forwardHow can you distinguish aldehydes, esters and carboxylic acids using IR spectra? Explain using specific examples.arrow_forward
- 2arrow_forward5.) Determine and draw the structure corresponding to the spectral information provided below. Explain what clues you used to determine the structure.arrow_forward1. How can you distinguish aldehydes, ketones, and carboxylic acids from each other using IR spectra? Explain, using specific examples. 2. How can you distinguish aldehydes, esters, and carboxylic acids using IR spectra? Explain, using specific examples. 3. How can you distinguish an alcohol, a primary amine, and a secondary amine using IR spectra? Explain, using specific examples. 4. How can you distinguish a terminal alkyne and a nitrile using IR spectra? Explain, using specific examples.arrow_forward
- a. Are any of the spectra that of an alcohol? If so, which? What absorption pattern(s) at what wavelength(s) identifies an alcohol? b. Are any of the spectra that of a compound containing a benzene ring? If so, which? What three absorption patterns at what wavelengths show that a compound has a benzene ring?arrow_forwardIndicate the functional groups found in the IR signals of: 1. Hexene 2. Cyclohexene 3. Toluene 4. Benzenearrow_forwardThe mass spectrum of compound C, with a molecular formula C8H7BrO2 is shown below. 1.Draw the most likely ion fragment for the signals at m/z 155, 159, 183, and 185.Explain why these signals appear as pairs with almost similar intensities in the mass spectrum. 2.Identify and draw the structure of compound C.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY