
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
9th Edition
ISBN: 9781292151229
Author: Wade, LeRoy G.
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.5, Problem 12.2P
Which of the bonds shown in red are expected to have IR-active stretching frequencies?
- (a) H—C≡C—H
- (b) H—C≡C—H
- (c) H—C≡C—CH3
- (d) H3C—C≡C—CH3
- (e) H3C—C≡C—CH3
- (f) H3C—CH3
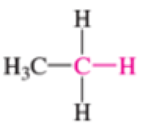
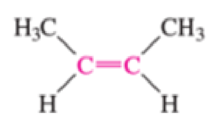
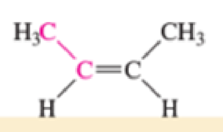
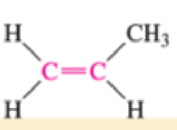
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
5. What are the spin system of the following compounds:
(a)
Cl₂CHCH2CHC₁₂
(b) CF3C=C-H
(c)
CI-
H
H
H
H
-Br
(d)
H3C
H
CH3
9. Consider the ethyl iodide molecule, CH CH₂, which of the statements below is correct?
(A) CH, protons are more deshielded.
(B) The energy gap between a and ß spin states of CH₂ protons is smaller than the counterpart of
CH; protons.
(C) The electronegative element I shields the CH₂ protons.
(D) None of the above.
Q 1(a)
Methane, CH4 is a tetrahedral molecule and does not exhibit a permanent dipole
moment (i.e. it has a dipole moment of 0). Nonetheless, in microwave spectroscopy
methane does exhibit a weak, but measurable absorbance. Explain why you think this
is the case?
Q 1(b)
The diatomic molecule 12C32S has been detected in interstellar gas clouds by
microwave spectroscopy. The masses of the two atoms are C = 12.00 amu and S=
31.972 amu and 12C32S has an equilibrium bond length of 1.534 Å.
Predict which rotational transition in 12C32S will have the greatest population at a
temperature of 85 K.
Chapter 12 Solutions
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Without doing the calculation, which member of each pair do you expect to occur at the higher frequency? (a) C=O or (b) (c) C=C stretching C=O C=C C=O or C-O stretching C=O -0 C=C or C-O stretching O O C=C C=O (d) C-H or C-C1 stretching C-H C-CIarrow_forwardWhich would you expect to have higher frequency C=O stretching vibration? H ہو H H H —F Farrow_forwardThe absorption pattern in the UV/VIS region corresponds to (a) bond vibrations b) valance electron transitions =) molecular rotations E) nuclear spin 0000 a an IR spectrumarrow_forward
- 13C NMR spectroscopy provides valuable information about the environments of a molecule's carbon atoms. Since carbon atoms are often connected to hydrogen atoms, which could split the carbon signal through spin-spin coupling, the coupling between C and H is often "turned off" through the use of broadband decoupling, causing each C signal to appear as a singlet. Draw an isomer of C5H11Cl that would be expected to have four resonances in its 13C NMR spectra.arrow_forwardA typical 1"B – 'H bond has a stretching frequency of 2400 cm1. Based on your understanding of isotopic effects on vibrational frequencies, and their connection to bond strength, rank the B - H bonds from weakest to strongest. Consider the most common isotopes of the B- H bonds highlighted below. Make sure to explain your ranking. 10В — 1н, 10В — ?н, 11в — 1Н, 11В - нarrow_forward2. Provide the approximate wavenumber for the IR absorption band from stretching vibration of bond identified in each structure. || -CEN H TIL H javarrow_forward
- 2. Which of the following compounds would you expect to have the highest infrared absorption frequency C-X bond? Bubble in your answer completely. O Br O O Oarrow_forwardFind the correct order of stretching frequency of O-O bond in the following molecules: O2, 02(-), 02(+) * 02 > 02 (+) > 02 (-) 02 (+) > 02 > 02 (-) O 02 (-) > 02 > 02 (+) O 02 > 02 (-) > 02 (+)arrow_forward(b) Identify the spin systems (AB, AX, AMX, etc.) constituted by H *s in the following compounds : H" CI Сно NCarrow_forward
- which of the following compounds absorb the radiation at longer wave length, Why ? 1- H;C-CH CH-CH=CH-CH3 2- H,C CH CH-CH=CH-C-CH3arrow_forwardWhich molecules below are rotational Raman active and which are inactive. O3 CH4 NH3 H2 H2O SiH4 CF6 C4H10arrow_forwardCalculate the energy(kJ)whenphotons areejected from 3molesofhydrogen atomsupon an electron transition from n = 4to n = 2. ∆?=−??(1??2−1??arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY