
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
9th Edition
ISBN: 9781292151229
Author: Wade, LeRoy G.
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.14B, Problem 12.7P
Identify which of these four mass spectra indicate the presence of sulfur, chlorine, bromine, iodine, or nitrogen. Suggest a molecular formula for each.
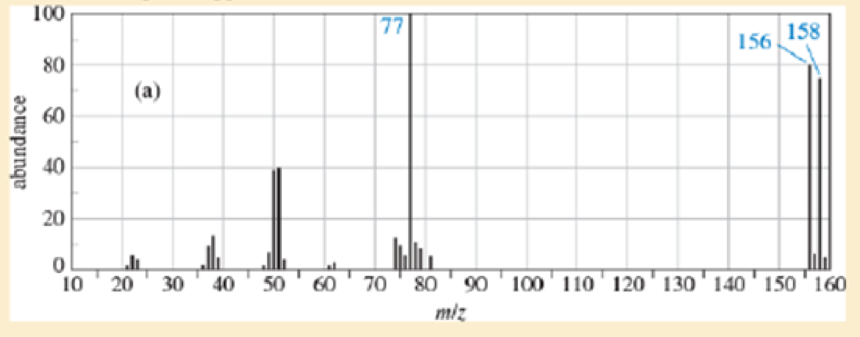
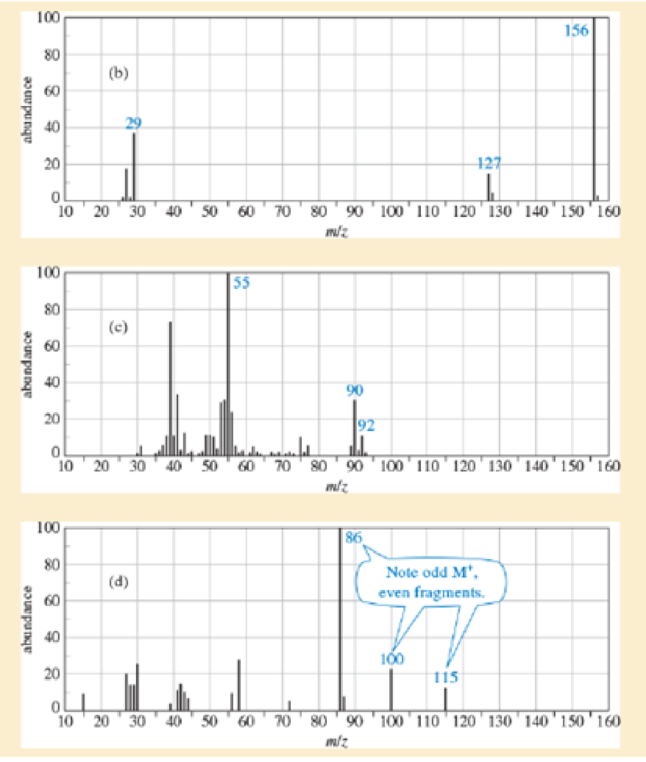
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
The atomic mass of fluorine is 18.998 amu, and its mass spectrum shows a large peak at this mass. The atomic mass of chlorine is 35.45 amu, yet the mass spectrum of chlorine does not show a peak at this mass. Explain the difference.
The mass spectrum of an organic compound shows the relative abundances of M to be 87.83% and M+1 to be 11.40%.
Assuming the peaks are caused by 12C and 1C isotopes, determine the number of carbon atoms in the compound. The
natural abundance of 1?C is 98.93%, and the natural abundance of 13C is 1.07%.
number of carbon atoms:
How many peaks would be observed on a mass spectrum for H2S+ ion? Hydrogen has two stable isotopes, 1H and 2H, and sulfur has 4 stable isotopes, 32S, 33S, 34S, and 36S. Assume that the ion does not decompose into smaller fragments.
Chapter 12 Solutions
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Boron consists of two isotopes, 10B and 11B. Chlorine also has two isotopes, 35Cl and 37Cl. Consider the mass spectrum of BC13. How many peaks would be present, and what approximate mass would each peak correspond to in the BCl3 mass spectrum?arrow_forwardThe mass spectrum of phosphoryl chloride. POF3, is illustrated here. (a) Identify the cation fragment at a m/Z ratio of 85. (b) Identify the cation fragment at a m/Z ratio of 69. (c) Which two peaks in the mass spectrum provide evidence that the oxygen atom is connected to the phosphorus atom and is not connected to any of the three fluorine atoms?arrow_forward2.75 Chlorine has only two isotopes, one with mass 35 and the other with mass 37. One is present at roughly 75% abundance, and the atomic weight of chlorine on a periodic table is 35.45. Which must be the correct mass spectrum for chlorine?arrow_forward
- The mass spectrum of CH3Cl is illustrated here. You know that carbon has two stable isotopes, and 13C with relative abundances of 98.9% and 1.1%, respectively, and chlorine has two isotopes, 35a and 37CI with abundances of 75.77% and 24.23%, respectively. (a) What molecular species gives rise to the lines at m/Z of 50 and 52? Why is the line at 52 about 1/3 the height of the line at 50? (b) What species might be responsible for the line at m/Z = 51?arrow_forwardGallium arsenide, GaAs, has gained widespread use in semiconductor devices that convert light and electrical signals in fiber-optic communications systems. Gallium consists of 60.% 69Ga and 40.% 71Ga. Arsenic has only one naturally occurring isotope, 75As. Gallium arsenide is a polymeric material, but its mass spectrum shows fragments with the formulas GaAs and Ga2As2. What would the distribution of peaks look like for these two fragments?arrow_forwardThe element bromine is Br2, so the mass of a Br2 molecule is the sum of the mass of its two atoms. Bromine has two isotopes. The mass spectrum of Br2 produces three peaks with relative masses of 157.836, 159.834, and 161.832, and relative heights of 6.337, 12.499. and 6.164, respectively. (a) What isotopes of bromine are present in each of the three peaks? (b) What is the mass of each bromine isotope? (c) What is the average atomic mass of bromine? (d) What is the abundance of each of the two bromine isotopes?arrow_forward
- Consider the following data for three binary compounds of hydrogen and nitrogen: %H (by Mass) %N (by Mass) I 17.75 82.25 II 12.58 87.42 III 2.34 97.66 When 1.00 L of each gaseous compound is decomposed to its elements, the following volumes of H2(g) and N2(g) are obtained: H2(L) N2(L) I 1.50 0.50 II 2.00 1.00 III 0.50 1.50 Use these data to determine the molecular formulas of compounds I, II, and III and to determine the relative values for the atomic masses of hydrogen and nitrogen.arrow_forward3.116 The simplest approximate chemical formula for the human body could be written as C728H4850O1970N104Ca24P16K4S4Na3Cl2Mg. Based on this formula, describe how you would rank by mass the ten most abundant elements in the human body.arrow_forwardMass spectrometric analysis showed that there are four isotopes of an unknown element having the following masses and abundances: Three elements in the periodic table that have atomic weights near these values are lanthanum (La), atomic number 57, atomic weight 138.9055; cerium (Ce), atomic number 58, atomic weight 140.115; and praseodymium (Pr), atomic number 59, atomic weight 140.9076. Using the data above, calculate the atomic weight, and identify the element if possible.arrow_forward
- 2.74 The accompanying table provides the identity of the two naturally occurring isotopes for four elements and the atomic weights for those elements. (In each case, the two isotopes differ in mass number by two.) Which element has the mass spectrum shown? Explain your answer.arrow_forwardThe action of bacteria on meat and fish produces a compound called cadaverine. As its name and origin imply, it stinks! (It is also present in bad breath and adds to the odor of urine.) It is 58.77% C, 13.81% H, and 27.40% N. Its molar mass is 102.2 g/mol. Determine the molecular formula of cadaverine.arrow_forwardEarly tables of atomic weights (masses) were generated by measuring the mass of a substance that reacts with 1.00 g of oxygen. Given the following data and taking the atomic mass of hydrogen as 1.00, generate a table of relative atomic masses for oxygen, sodium, and magnesium. Element Mass That Combines with 1.00g Oxygen Assumed Formula Hydrogen 0.126 g HO Sodium 2.875 g NaO Magnesium 1.500 g MgO How do your values compare with those in the periodic table? How do you account for any differences?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY