
Concept explainers
(a)
Interpretation:
The mechanism for the reaction that will take place when the given compound is treated with
Concept introduction:
Diazomethane,
The stereochemistry of the double bond is preserved in the addition. If the double bond has a cis relationship, then the two substituents end up on the same side of the cyclopropane ring. If the two substituents on the double bond are trans to each other, they end up on the opposite sides of the cyclopropane ring. This also determines the product distribution if the cyclic product is chiral. If the starting
Answer to Problem 12.26P
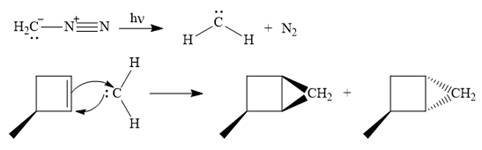
The mechanism for the reaction when
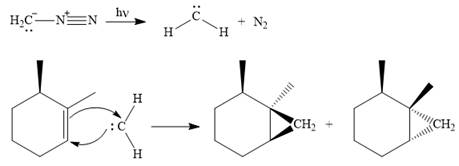
Explanation of Solution
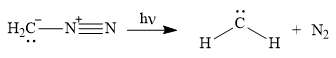
Diazomethane dissociates heterolytically when irradiated with ultraviolet light to produce a carbene and a nitrogen molecule.
The carbene then simultaneously adds to the initially double-bonded carbons. The lone pair on the carbene forms a bond with one carbon while the
Since the starting alkene is chiral, the product will be a mixture of two enantiomers. The pair of enantiomers is produced because the carbene can add either above or below the plane of the ring in the reactant.
Thus the complete mechanism for this reaction can be drawn as
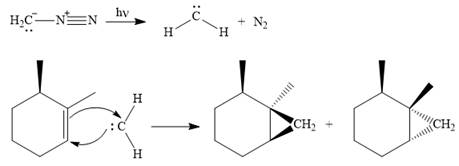
A carbene adds to an alkene or
(b)
Interpretation:
The mechanism for the reaction that will take place when the given compound is treated with
Concept introduction:
Diazomethane,
The stereochemistry of the double bond is preserved in the addition. If the double bond has a cis relationship, then the two substituents end up on the same side of the cyclopropane ring. If the two substituents on the double bond are trans to each other, they end up on the opposite sides of the cyclopropane ring. This also determines the product distribution if the cyclic product is chiral. If the starting alkene is achiral and the product chiral, the product is a racemic mixture of enantiomers. If the starting alkene is also chiral, then an unequal mixture of enantiomers is produced.
Answer to Problem 12.26P
The mechanism for the given reaction is
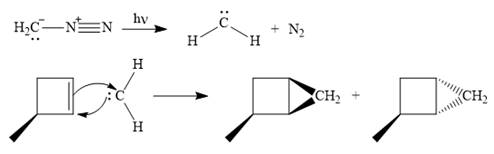
Explanation of Solution
Diazomethane dissociates heterolytically when irradiated with ultraviolet light to produce a carbene and a nitrogen molecule.

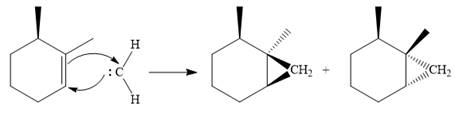
The carbene then simultaneously adds to the initially double-bonded carbons. The lone pair on the carbene forms a bond with one carbon while the
The carbene then simultaneously adds to the initially double-bonded carbons. The lone pair on the carbene forms a bond with one carbon while the
Since the starting alkene is chiral, the product will be a mixture of two enantiomers. The pair of enantiomers is produced because the carbene can add either above or below the plane of the ring in the reactant.
Thus the complete mechanism for this reaction can be drawn as:
A carbene adds to an alkene or alkyne to produce a cyclopropane ring.
(c)
Interpretation:
The mechanism for the reaction that will take place when the given compound is treated with
Concept introduction:
Diazomethane,
The stereochemistry of the double bond is preserved in the addition. If the double bond has a cis relationship, then the two substituents end up on the same side of the cyclopropane ring. If the two substituents on the double bond are trans to each other, they end up on the opposite sides of the cyclopropane ring. This also determines the product distribution if the cyclic product is chiral. If the starting alkene is achiral and the product chiral, the product is a racemic mixture of enantiomers. If the starting alkene is also chiral, then an unequal mixture of enantiomers is produced.
Answer to Problem 12.26P
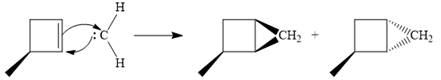
The mechanism for the given reaction is
Explanation of Solution
Diazomethane dissociates heterolytically when irradiated with ultraviolet light to produce a carbene and a nitrogen molecule.
![]()
The carbene then simultaneously adds to the initially double-bonded carbons. The lone pair on the carbene forms a bond with one carbon while the
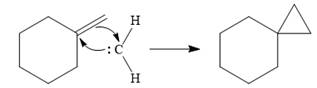
Only one product is formed in this case because of the symmetry of the reactant alkene about the axis of the double bond.
Thus, the complete mechanism for the given reaction can be drawn as
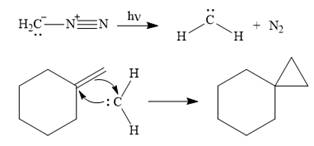
A carbene adds to an alkene or alkyne to produce a cyclopropane ring.
(d)
Interpretation:
The mechanism for the reaction that will take place when the given compound is treated with
Concept introduction:
Diazomethane,
The stereochemistry of the double bond is preserved in the addition. If the double bond has a cis relationship, then the two substituents end up on the same side of the cyclopropane ring. If the two substituents on the double bond are trans to each other, they end up on the opposite sides of the cyclopropane ring. This also determines the product distribution if the cyclic product is chiral. If the starting alkene is achiral and the product chiral, the product is a racemic mixture of enantiomers. If the starting alkene is also chiral, then an unequal mixture of enantiomers is produced.
Answer to Problem 12.26P
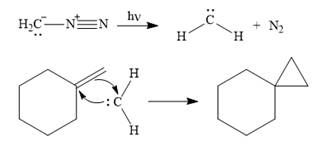
The mechanism for the given reaction is
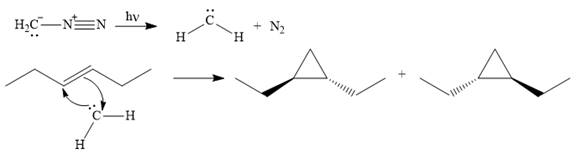
Explanation of Solution
Diazomethane dissociates heterolytically when irradiated with ultraviolet light to produce a carbene and a nitrogen molecule.
![]()
The carbene then simultaneously adds to the initially double-bonded carbons. The lone pair on the carbene forms a bond with one carbon while the
The starting alkene has a trans geometry, therefore, the two substituents on the cyclopropane ring in the product are on opposite sides of the ring.
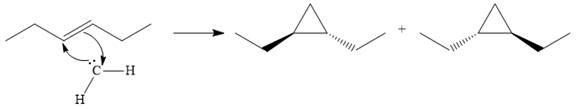
This means the product is chiral, and a racemic mixture of two enantiomers will be produced.
Thus, the complete mechanism for the reaction can be drawn as

A carbene adds to an alkene or alkyne to produce a cyclopropane ring.
(e)
Interpretation:
The mechanism for the reaction that will take place when the given compound is treated with
Concept introduction:
Diazomethane,
The stereochemistry of the double bond is preserved in the addition. If the double bond has a cis relationship, then the two substituents end up on the same side of the cyclopropane ring. If the two substituents on the double bond are trans to each other, they end up on the opposite sides of the cyclopropane ring. This also determines the product distribution if the cyclic product is chiral. If the starting alkene is achiral and the product chiral, the product is a racemic mixture of enantiomers. If the starting alkene is also chiral, then an unequal mixture of enantiomers is produced.
Answer to Problem 12.26P
The mechanism for the given reaction is
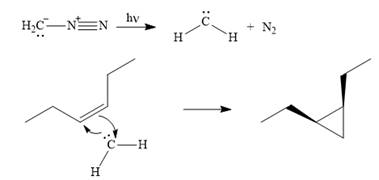
Explanation of Solution
Diazomethane dissociates heterolytically when irradiated with ultraviolet light to produce a carbene and a nitrogen molecule.
![]()
The carbene then simultaneously adds to the initially double-bonded carbons. The lone pair on the carbene forms a bond with one carbon while the
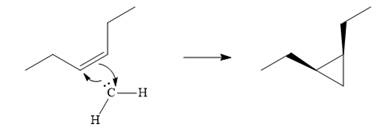
The starting alkene has a cis geometry and is symmetric. Therefore, only one product, a meso compound is produced.
Thus the complete mechanism for this reaction can be drawn as

A carbene adds to an alkene or alkyne to produce a cyclopropane ring.
Want to see more full solutions like this?
Chapter 12 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- Draw the least stable resonance form for the intermediate in the following electrophilic substitution reaction.arrow_forwardDraw the mechanisms and products for the following reactionsarrow_forwardDraw the structure for the major reaction product for the following reactions. Draw out the mechanism.arrow_forward
- Draw a reaction mechanism for this reactionarrow_forwardAdd curved arrows to draw step four of the mechanism. Modify the given drawing of the product as needed to show the intermediate that is formed in the step.arrow_forwardIf you answered "yes" for the first susbtrate, draw the intermediate that forms during a nucleophilic substitution reaction in the space below the table.arrow_forward
- Ethylene glycol can be used as a protecting group for ketones and aldehydes. Draw the mechanism for the following reaction.arrow_forwardFor each set of reactions, circle the mechanism (SN2 vs SN1), draw the main organic substitution/elimination product (for each reaction draw the product, though in some cases it may be equivalent) and indicate which reaction occurs at the faster rate.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





