
(a)
Interpretation:
The detailed mechanism with the major product for the given reaction is to be drawn.
Concept introduction:
The acid-catalyzed hydration of an
Answer to Problem 12.42P
The detailed mechanism for the given reaction is
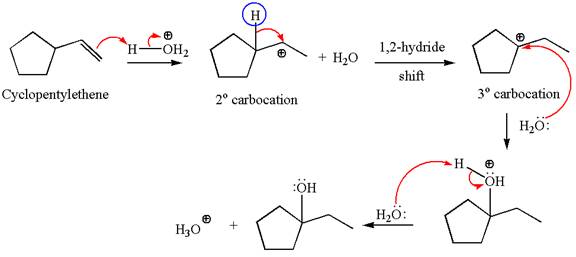
Explanation of Solution
The given reaction is
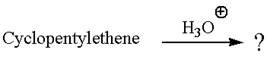
Cyclopentylethene, a terminal alkene, in presence of
The first step is the formation of a secondary carbocation by proton transfer reaction. The proton transfers to the less substituted double bonded carbon.
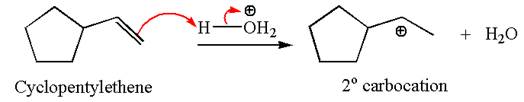
The secondary carbocation can be rearranged to a more stable tertiary carbocation by
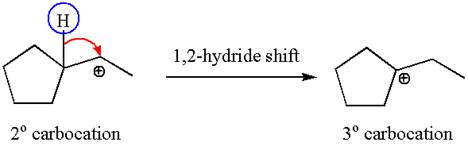
In the second step, the water molecule acts as a nucleophile and attacks the tertiary carbocation, forming a tertiary alcohol product, followed by deprotonation of the positively charged oxygen.
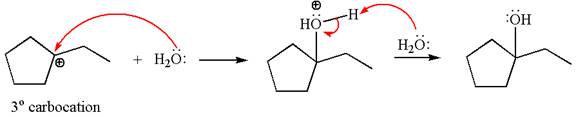
The detailed mechanism for the given reaction is drawn by suggesting that the reaction occurs through carbocation rearrangement.
(b)
Interpretation:
The detailed mechanism with major product for the given reaction is to be drawn.
Concept introduction:
The oxymercuration-reduction is also the reaction of addition of water across the
Answer to Problem 12.42P
The detailed mechanism for the given reaction is
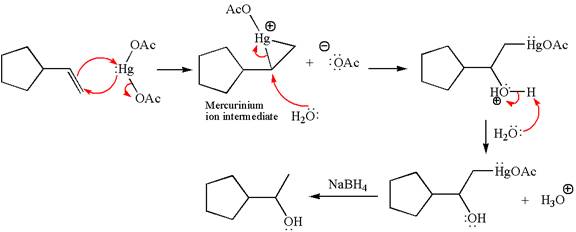
Explanation of Solution
The given reaction is
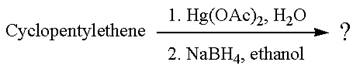
Cyclopentylethene, a terminal alkene, on reaction with mercury
In the first step, the electron rich
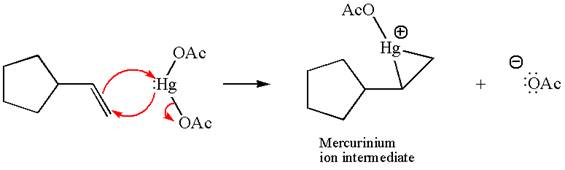
In the second step, the water molecule acts as a nucleophile and, according to Markovnikov rule, attacks the most substituted side to open the three-membered ring, followed by deprotonation of the positively charged oxygen atom.
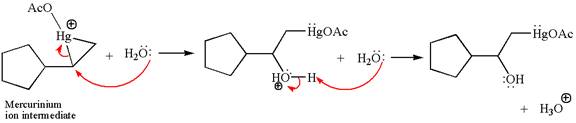
The product formed in the previous step is then subjected to reduction with sodium borohydride,
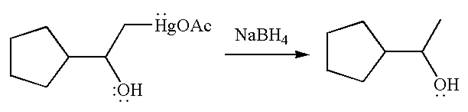
The detailed mechanism for the given reaction is drawn by suggesting that the reaction occurred through formation of mercurinium ion intermediate without rearrangement.
(c)
Interpretation:
The detailed mechanism with major product for the given reaction is to be drawn.
Concept introduction:
The acid-catalyzed hydration of an alkene is the electrophilic addition of water across the
Answer to Problem 12.42P
The detailed mechanism for the given reaction is
Explanation of Solution
The given reaction is
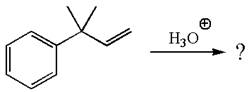
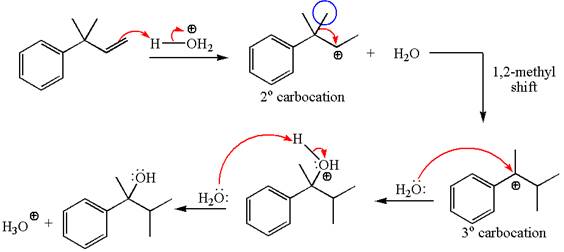
The given substrate, a terminal alkene, in the presence of
The first step is the formation of a secondary carbocation by proton transfer reaction. The proton transfers to the less substituted double bonded carbon.
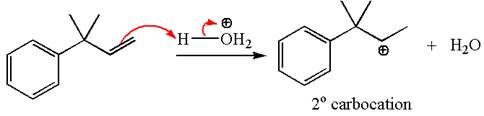
The secondary carbocation can be rearranged to a more stable tertiary as well as resonance stabilized carbocation by
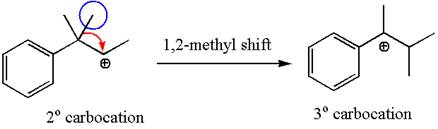
In the second step, the water molecule acts as a nucleophile and attacks the tertiary carbocation, forming a tertiary alcohol product, followed by deprotonation of the positively charged oxygen.
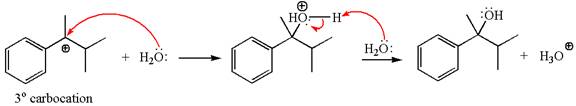
The detailed mechanism for the given reaction is drawn by suggesting that the reaction occurred through carbocation rearrangement.
(d)
Interpretation:
The detailed mechanism with major product for the given reaction is to be drawn.
Concept introduction:
The oxymercuration-reduction is also the reaction of addition of water across the
Answer to Problem 12.42P
The detailed mechanism for the given reaction is
Explanation of Solution
The given reaction is
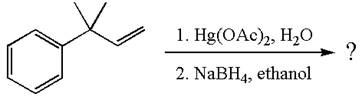
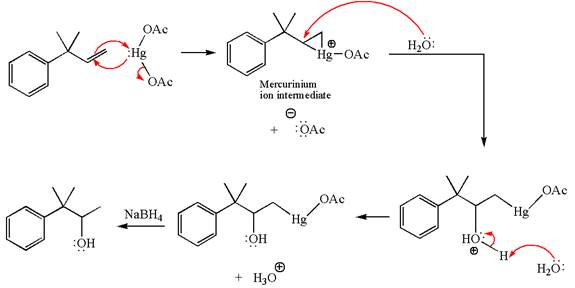
The given substrate, a terminal alkene, on reaction with mercury
In the first step, the electron rich
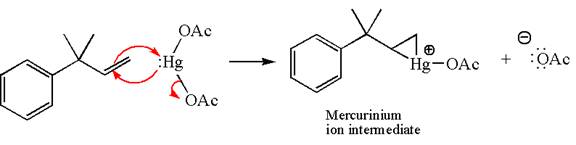
In the second step, the water molecule acts as a nucleophile and, according to Markovnikov rule, attacks the most substituted side to open the three-membered ring, followed by deprotonation of the positively charged oxygen atom.
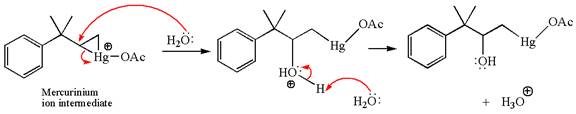
The product formed in the previous step is then subjected to reduction with sodium borohydride,
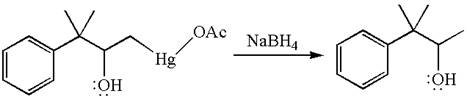
The detailed mechanism for the given reaction is drawn by suggesting that the reaction occurred through formation of mercurinium ion intermediate without rearrangement.
Want to see more full solutions like this?
Chapter 12 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- Draw the mechanism and the major organic product for each of the following reactions. Please show all work with arrowsarrow_forwarddraw all of the mechanism for this given acidarrow_forwardThe reaction shown here is a halosulfonation, which is a useful variation of the sulfonation reaction. Draw the complete mechanism for this reaction.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





