
Concept explainers
(a)
Interpretation:
The IUPAC name for the compound is to be stated.
Concept introduction:
The systematic naming of organic compound is given by
Rules for writing IUPAC name from structural formula are shown below.
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 12.4E
The name of compound is
Explanation of Solution
The compound is given as
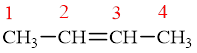
Figure 1
Therefore, the name of the compound is
The name of compound is
(b)
Interpretation:
The IUPAC name for compound is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are shown below.
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 12.4E
The name of the given compound is
Explanation of Solution
The compound is given as shown below.
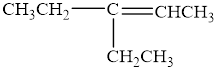
Figure 2
The parent chain is pentene, that is, it consists of five carbon atoms and a double bond between the second and the third carbon atoms. An ethyl group is present as a substituent on the third carbon atom as shown below.
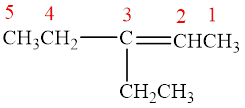
Figure 3
Therefore, the name of compound is
The name of compound is
(c)
Interpretation:
The IUPAC name for the given compound is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are shown below.
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 12.4E
The name of compound is
Explanation of Solution
The compound is given as shown below.
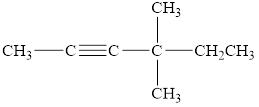
Figure 4
The parent chain is hexyne, that is, it consists of six carbon atoms and a triple bond between the second and the third carbon atoms. Two methyl groups are present as a substituent on the fourth carbon atom as shown below.
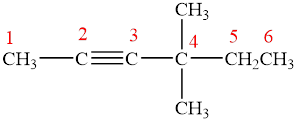
Figure 5
Therefore, the name of compound is
The name of compound is
(d)
Interpretation:
The IUPAC name for the given compound is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are shown below.
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 12.4E
The name of compound is
Explanation of Solution
The compound is given as shown below.
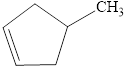
Figure 6
The parent chain is cyclopentene, that is, it consists of five carbon atoms in a ring and a double bond between the first and the second carbon atoms. A methyl group is present as a substituent on the fourth carbon atom as shown below.
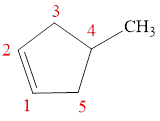
Figure 7
Therefore, the name of compound is
The name of compound is
(e)
Interpretation:
The IUPAC name for compound is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are shown below.
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 12.4E
The name of compound is
Explanation of Solution
The compound is given as shown below.
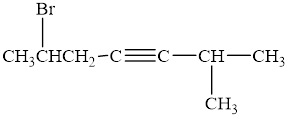
Figure 8
The parent chain is heptyne, that is, it consists of seven carbon atoms and a triple bond between the third and the fourth carbon atoms. A methyl group and a bromine group are present as substituents on the second and the sixth carbon atoms respectively as shown below.
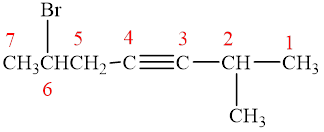
Figure 9
Therefore, the name of the given compound is
The name of compound is
(f)
Interpretation:
The IUPAC name for the given compound is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are shown below.
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 12.4E
The name of compound is
Explanation of Solution
The compound is given as shown below.
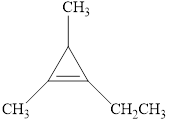
Figure 10
The parent chain is cyclopropene, that is, it consists of three carbon atoms and a double bond between the first and the second carbon atoms. An ethyl group and two methyl groups are present as a substituent on the first, second and the third carbon atoms respectively as shown below.
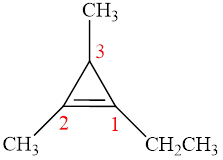
Figure 11
Therefore, the name of compound is
The name of compound is
(g)
Interpretation:
The IUPAC name for compound is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are shown below.
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 12.4E
The name of compound is
Explanation of Solution
The compound is given as shown below.
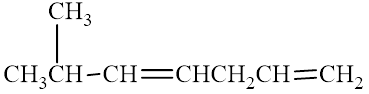
Figure 12
The parent chain is heptadiene, that is, it consists of seven carbon atoms and double bonds between the first, second and the fourth, fifth carbon atoms. A methyl group is present as a substituent on the sixth carbon atom as shown below.
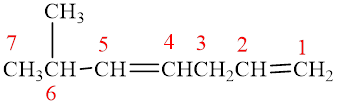
Figure 13
Therefore, the name of compound is
The name of compound is
Want to see more full solutions like this?
Chapter 12 Solutions
CHEMISTRY FOR TODAY+OWLV2 24 MO>IP<
- Isooctane is the common name of the isomer of C8H18 used as the standard of 100 for the gasoline octane rating: (a) What is the IUPAC name for the compound? (b) Name the other isomers that contain a five-carbon chain with three methyl substituents.arrow_forwardDraw the structures of the chief product formed when the following alcohols are dehydrated to alkenes: a. b.arrow_forwarda. Name each of the following alcohols. b. Name each of the following alcohols, including the stereochemistry if cis-trans isomers are possible.arrow_forward
- Name and draw a structural formula for the product of each alkene addition reaction. (a) (b)arrow_forward1. Write down the structural formulas for three isomeric compounds containing the carbonyl group and having the molecular formula C4H8O. Then give it the IUPAC name. 3. What is the product of the following reaction?arrow_forwardGive the IUPAC name for each alkene.arrow_forward
- Give the IUPAC name for the alkyl halide below.arrow_forwardALCOHOLS 1. WHY IS ETHANOL MORE SOLUBLE IN WATER THAN 1-HEXANOL? 2. WHAT IS DENATURED ALCOHOL? AND WHY IS ALCOHOL DENATURED? ETHER 1. WHY DOES DIETHYL ETHER HAVE MUCH LOWER BOILING POINT THAN 1-BUTANOL?arrow_forwardGive the IUPAC name of the attached alkyl halide ?arrow_forward
- 3. a. What is the chemical structure of benzoic acid, circle functional groups different than alkane,alkene, alkyne? b. Is it polar or nonpolar? _______________________ c. What is its water solubility in g/L? __________________________arrow_forwardComplete the IUPAC Name of the structure.arrow_forwardwhat is the IUPAC name for this? step by step plsarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





