
Concept explainers
Interpretation:
The number of tertiary carbons present has to be determined in the compound as shown in Problem 12.65.
Concept introduction:
Classification of Carbon atom:
Carbons can be classified in to three types,
Primary,
Secondary,
Tertiary
Quaternary
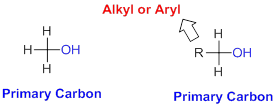
Primary Carbon:
Carbon that directly attached to three or two hydrogens having one or two (alkyl or aryl) respectively is primary carbon.
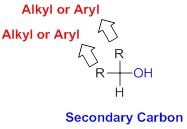
Secondary Carbon:
Carbon is directly attached with one hydrogen atoms and two alkyl or aryl group (two substituents) is called Secondary Carbon.
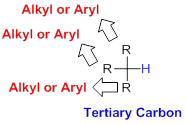
Tertiary Carbon:
Carbon is directly attached to three alkyl or aryl groups (three substituents) is called
Quaternary Carbon:
Carbon is directly attached to four alkyl or aryl groups (four substituents) is called 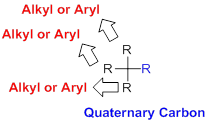
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
- Trehalose, a disaccharide found in the blood of insects, has the following structure. What simple sugars would you obtain on hydrolysis of trehalose?arrow_forwardGiven the balanced equation with an unknown compound represented by X, which compound is represented by X?arrow_forwardDraw a Fischer projection formula for the enantiomer of each of the following monosaccharides. (a to d)arrow_forward
- Which of the following Fischer formula is or are monosaccharide that has two chiral centers?arrow_forwardIf the phosphorus atom in 3-phosphoglycerate is radioactively labeled, where will the label be when the reaction that forms 2-phosphoglycerate is over?arrow_forwardWhy is it not possible to find a five-fold symmetry operation in naturally-occurring crystals?arrow_forward
- What is the molecular formula of glucose? How can its structural formula be described?arrow_forwardCan there be any chiral carbon atoms in triacylglycerols? If so, which ones can be chiral and what determines their chirality?arrow_forwardIf one compound has the formula C5H10 and another has the formula C4H10, are the two compounds isomers? Explain.arrow_forward
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
