
Concept explainers
Interpretation:
It is to verify that the
Concept introduction:
Bond energy is the measure of bond strength in a chemical bond. In a compound, the nuclei are bind together by
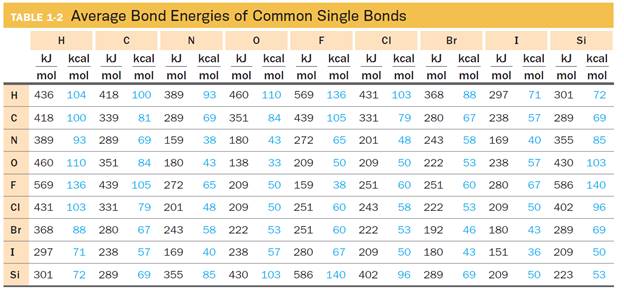
Table 1-2 lists average bond energies for a variety of common bonding partners found in organic species and contains only single bonds:
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
ORGANIC CHEM. PRIN AND MECHANISM LL+ AC
- Use curved arrows to show the most likely acid-base reaction for each pair of reactants below.Draw the resulting products including all important resonance structures. For each reaction, indicate if Hrxn is negative, positive, or close to zero, and explain your reasoning.arrow_forwardYou will not find “hydroxide” in the stockroom, but you will find sodium hydroxide (NaOH) andpotassium hydroxide (KOH). Lithium hydroxide (LiOH) is expensive and used in spacecraft airfilters since hydroxide reacts with carbon dioxide, and lithium is lighter than sodium or potassium.Cesium and francium hydroxides are very expensive and little used. Is this information consistentwith your answer to the previous question?arrow_forwardFor NH3 (ammonia) and H2O (water)... a. Use curved arrows to show the most likely acid-base reaction, and draw the resultingproducts. (Hint: First decide which is the stronger acid, and which is the stronger base.) b. Mark each curved arrow with a positive (bond-breaking) or negative (bond-forming) numberindicating the energy change associated with that arrow (in pKa units). c. Calculate Hrxn and write this number above a set of reaction arrows that indicate whichdirection is downhill/favorable (in the example, the reaction is downhill to the right). d. Sketch an energy diagram for the reaction. e. Is your energy diagram consistent with the fact that, in this case, the most likely acid-basereaction is endothermic?arrow_forward
- Add a + or above each curved arrow in Figure 4.11 to show the sign of the energy change.arrow_forwardIs bond formation endothermic or exothermic? Write a + or sign above the arrow in the previousquestion to represent the sign of the energy change associated with the arrow.arrow_forward13. Aniline and nitrobenzene are two substituted benzene compounds that contain nitrogen atoms. A) Use resonance structures to identify all carbon atoms that are electron rich on aniline. Mark the appropriate carbons in the figure below with a d-. NH₂ nitrobenzene aniline Note: this is benzene B) Use resonance structures to identify all carbon atoms that are electron deficient on nitrobenzene. Mark the appropriate carbons in the figure below with a dª.arrow_forward
- Add formal charges to each resonance form of HCNO. Resonance structure A Select Draw Rings More / Ć H / C Resonance structure C N -C-N=o: Select Draw Rings More |||||| H H-C= NOH Q2 Q Erase : 0: Erase Resonance structure B Select Draw Rings H с More N O ▬▬▬▬▬▬▬▬▬▬▬▬ H Erase Q2 Q Based on the formal charges you added, which structure is favored? A B Carrow_forwardThis one is a little different, but can u answer it as wellarrow_forward! ( give ans with explanation ) if u hv answerd earlier plz do not answer because earlier answer was wrong. give only correct answer with explanationarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
