
(a)
Interpretation:
Concept introduction:To remove the electron situated in outermost shell certain minimum energy must be imparted so as to convert an atom to gaseous species. The energy thus imparted represents ionization energy.
The magnitude of ionization energy is determined by how effectively valence electron is held by the nucleus. If the outermost shell has, for instance, one or two electronsthat require very minimum ionization energy because they can attain the noble gas configuration upon loss of those electrons.
(a)
Explanation of Solution
With 2 electrons with
(b)
Interpretation:Electrons in
Concept introduction:To remove the electron situated in outermost shell certain minimum energy must be imparted so as to convert an atom to gaseous species. The energy thus imparted represents ionization energy.
The magnitude of ionization energy is determined by how effectively valence electron is held by the nucleus. If the outermost shell has, for instance, one or two electronsthat require very minimum ionization energy because they can attain the noble gas configuration upon loss of those electrons.
(b)
Explanation of Solution
With 2 electrons with
(c)
Interpretation:Electrons in
Concept introduction:To remove the electron situated in outermost shell certain minimum energy must be imparted so as to convert an atom to gaseous species. The energy thus imparted represents ionization energy.
The magnitude of ionization energy is determined by how effectively valence electron is held by the nucleus. If the outermost shell has, for instance, one or two electronsthat require very minimum ionization energy because they can attain the noble gas configuration upon loss of those electrons.
(c)
Explanation of Solution
With 2 electrons with
(d)
Interpretation:Electrons in
Concept introduction:To remove the electron situated in outermost shell certain minimum energy must be imparted so as to convert an atom to gaseous species. The energy thus imparted represents ionization energy.
The magnitude of ionization energy is determined by how effectively valence electron is held by the nucleus. If the outermost shell has, for instance, one or two electronsthat require very minimum ionization energy because they can attain the noble gas configuration upon loss of those electrons.
(d)
Explanation of Solution
With 2 electrons with
(e)
Interpretation:Number of neutrons in this element should be identified.
Concept introduction:To remove the electron situated in outermost shell certain minimum energy must be imparted so as to convert an atom to gaseous species. The energy thus imparted represents ionization energy.
The magnitude of ionization energy is determined by how effectively valence electron is held by the nucleus. If the outermost shell has, for instance, one or two electronsthat require very minimum ionization energy because they can attain the noble gas configuration upon loss of those electrons.
(e)
Explanation of Solution
With 2 electrons with
The formula to compute neutrons from mass number is as follows:
Atomic number is 24.
Mass number is 52.
Substitute the value in above formula.
So there are 28 neutrons in chromium.
(f)
Interpretation:Mass of
Concept introduction:To remove the electron situated in outermost shell certain minimum energy must be imparted so as to convert an atom to gaseous species. The energy thus imparted represents ionization energy.
The magnitude of ionization energy is determined by how effectively valence electron is held by the nucleus. If the outermost shell has, for instance, one or two electronsthat require very minimum ionization energy because they can attain the noble gas configuration upon loss of those electrons.
(f)
Explanation of Solution
With 2 electrons with
Since molar mass of chromium ion is
Thus, mass of
(g)
Interpretation:Ground-state electron configuration of neutral chromium should be written.
Concept introduction:Aufbau rule states that electrons must be filled in lowest energy levels first. For instance, electrons first occupy shells that are lower in energies illustrated as follows:
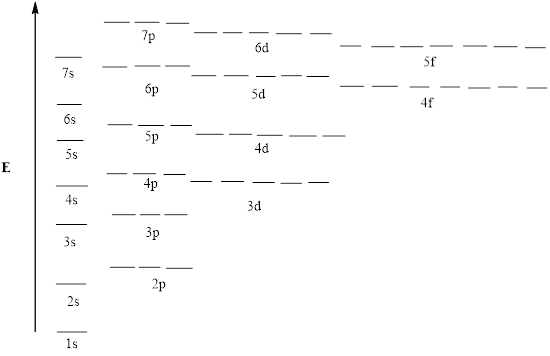
Pauli’s exclusion principle states thatno two or more than two electrons of a poly electron atom can have same values of 4 quantum numbers that are
Hund’s rule of maximum multiplicity states that electrons cannot be allowed to pair until each orbital gets singly filled with one electron. These 3 principles form basis for determination of electronic configuration.However, certain elements that are able to achieve nearest half-filled or fully filed configuration show exceptional configurations.
(g)
Explanation of Solution
With 2 electrons with
With atomic number as 24, expected configuration for
Want to see more full solutions like this?
Chapter 12 Solutions
Chemical Principles
- The periodic table consists of four blocks of elements that correspond to s, p, d, and f orbitals being filled. After f orbitals come g and h orbitals In theory, if a g block and an h block of elements existed, bow long would the rows of g and h elements be in this theoretical periodic table?arrow_forwardSuppose that the spin quantum number could have the values 12,0 and 12 . Assuming that the rules governing the values of the other quantum numbers and the order of filling sublevels were unchanged, (a) what would be the electron capacity of an s sublevel? a p sublevel? a d sublevel? (b) how many electrons could fit in the n=3 level? (c) what would be the electron configuration of the element with atomic number 8? 17?arrow_forwardAnswer the following questions, assuming that ms, could have three values rather than two and that the rules for n, l, and ml are the normal ones. a. How many electrons would an orbital be able to hold? b. How many elements would the first and second periods in the periodic table contain? c. How many elements would be contained in the first transition metal series? d. How many electrons would the set of 4f orbitals be able to bold?arrow_forward
- r Questions 11—13, you will need to consider ionizations beyond the first ionization energy. For example, the second ionization energy is the energy to remove a second electron from an element. Compare the first ionization energy of helium to its second ionization energy, remembering that both electrons come from the 1s orbital. l> X Y First 170 200 second 350 400 Third 1800 3500 fouth 2500 5000 entify the elements X and Y. There may be more than one answer. so explain completely.arrow_forwardConsider the orbitals shown here in outline. (a) What is the maximum number of electrons contained in an orbital of type (x)? Of type (y)? Of type (z)? (b) How many orbitals of type (x) are found in a shell with n=2? How many of type (y)? How many of type (z)? (c) Write a set of quantum numbers for an electron in an orbital of type (x) in a shell with n=4, of an orbital of type (y) in a shell with n=2. Of an orbital of type (z) in a shell with n=3. (d) What is the smallest possible n value for an orbital of type (x)? Of type (y)? Of type (z)? (e) What are the possible I and ml values for an orbital of type (x)? Of type (y)? Of type (z)?arrow_forwardWhich atom would be expected to have a half-filled 4s subshell?arrow_forward
- Answer the following questions: (a) Without using quantum numbers, describe the differences between the shells, subshells, and orbitals of an atom. (b) How do the quantum numbers of the shells, subshells, and orbitals of an atom differ?arrow_forwardWhat type of electron orbital (i.e., s, p, d, or f) is designated by an electron with quantum numbers (a) n=1,l=0,m l =0(b) n=3,l=2,m l =1? (c) n=4,l=3,m l =3arrow_forwardWhat evidence do we have that energy levels in an atom are quantized? State and explain the evidence.arrow_forward
- The first ionization energies of As and Se are 0.947 and 0.941 MJ/mol, respectively. Rationalize these values in terms of electron configurations.arrow_forwardUntil recently, it was thought that Ca was unstable, and that the Ca atom therefore had a negative electron affinity. Some new experiments have now measured an electron affinity of +2.0kJmol1 for calcium. What is the longest wavelength of light that could remove an electron from Ca ? In which region of the electromagnetic spectrum does this light fall?arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





