
Concept explainers
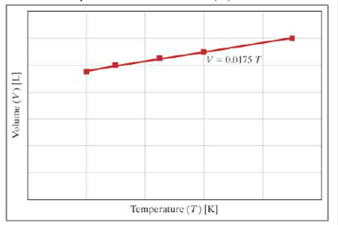
The graph shows the ideal gas law relationship (PV =nRT) between volume (V) and temperature (T).
- a. What are the units of the slope (0.0175)?
- b. If the tank has a pressure of 2.4 atmospheres and is filled with nitrogen (formula, N2; molecular weight, 28 grams per mole), what is the mass of gas in the tank in units of grams?
- c.
 If the tank is filled with 20 grams of oxygen (formula, o2; molecular weight, 32 grams per mole), what is the pressure of the tank (P) in units of atmospheres?
If the tank is filled with 20 grams of oxygen (formula, o2; molecular weight, 32 grams per mole), what is the pressure of the tank (P) in units of atmospheres?
a.
Find the slope units.
Answer to Problem 1ICA
The units of slope is
Explanation of Solution
Calculation:
Refer to the graph in the respective question. The expression is,
Rearrange the expression.
From the graph, the unit of volume (V) is L and temperature (T) is K. Therefore, the unit of slope (0.0175) is
Conclusion:
Thus, the units of slope is
b.
Find the mass of gas in terms of grams.
Answer to Problem 1ICA
The mass of nitrogen gas in terms of grams is 14.4 g.
Explanation of Solution
Given data:
Molecular weight (MW) of nitrogen is 28 grams per mole.
Pressure (P)is 2.4 atmospheres.
Formula used:
Consider the ideal gas law relationship.
Here,
Calculation:
Refer to the graph in the respective question. The expression is,
Modify equation (1) as follows.
Substitute
Substitute 2.4 atm for P and
Consider the general expression for mass in terms of molecular weight.
Substitute
Conclusion:
Thus, the mass of nitrogen gas in terms of grams is 14.4 g.
c.
Find the pressure of tank in terms of atmospheres.
Answer to Problem 1ICA
The pressure of tank in terms of atmospheres is 3.0 atm.
Explanation of Solution
Given data:
Molecular weight (MW) of oxygen is 32 grams per mole.
Mass of oxygen (m)is 20 grams.
Calculation:
Refer to the graph in the respective question.
Modify equation (2).
Substitute 20 g for
Modify equation (1).
Substitute
Substitute 0.625 mol for n and
Conclusion:
Thus, the pressure of tank in terms of atmospheres is 3.0 atm.
Want to see more full solutions like this?
Chapter 12 Solutions
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
- The below results are for cooling tower expirement, energy balance :energy balance of the system, that is to say, the energy taken by the air must be equal to the energy supplied by the water flow. Question : Discuss the tabulated cooling tower results... Discuss in detail format!arrow_forwardSubject : Combustion Engineering Please provide schematic diagram and complete solutions with formulas. A 650 BHP diesel engine uses fuel oil of 28 degree API gravity, fuel consumption is 0.65 lb/Bhp-hr. Cost of fuel is P7.95/L. Determine the minimum volume of cubical day tank in cu.cm, ambient temperature is 45 degrees C.arrow_forwardTHERMODYNAMICS UPVOTE WILL BE GIVEN. PLEASE WRITE THE COMPLETE SOLUTIONS/DIAGRAMS LEGIBLY. NO LONG EXPLANATION NEEDED. USE 3 DECIMAL PLACES. BOX THE FINAL ANSWER. A pump discharges into a semi spherical tank at the bottom and cylindrical on top with a radius of 2 meters, and a total tank height of 8 meters. The flow rate is 80 gallons per minute and fluid specific gravity= 1.1. Determine the following: A) flow rate in kg/sec and time it takes to fill the tank in hours. Flow Rate and Timearrow_forward
- Engineering Economy A company has determined the price and the monthly demand of its products are related by the equation D = √775 - p, where p is the price per unit in dollar and D, is the monthly demand. The associated fixed costs are $2,125 per month and the variable costs are $100/ unit. Use this information to answer the following: a)What is the optimal number of units that should be produced and sold each month? b) What is the maximum profit? c)Determine the value of D that represents the break-even point?arrow_forwardTHERMODYNAMICS UPVOTE WILL BE GIVEN. PLEASE WRITE THE COMPLETE SOLUTIONS/DIAGRAMS LEGIBLY. NO LONG EXPLANATION NEEDED. USE 3 DECIMAL PLACES. BOX THE FINAL ANSWER. An airplane is cruising at an elevation of 35,000 feet from sea level. Determine the amount of gage pressure in bars needed to pressurize the airplane to simulate sea level conditions. The average atmospheric pressure on earth is mated as a function of altitude by the relation Patm = 101.325 (1- 0.02256z)5.256, where Patm is the atmospheric pressure in kPa and z is the altitude in km with z = 0 at sea level.arrow_forwardThe following data are given for a biogas digester suitable for the output of six cows. The volume of digester is 8.3 m3 , the volume of gas holder is 2.2 m3 and the retention time is 22 days. Find the height of gas holder in centimeter.arrow_forward
- Make the graphs shown on the picture exactly the same. Follow the directions in the other picture. Please make the graphs look exactly the same as the picture. Make them on MATLAB, please send code.arrow_forwardTHERMODYNAMICS (ALREADY ANSWERED IN BARTLEBY. PLEASE CHECK IF THEIR ANSWER IS CORRECT) UPVOTE WILL BE GIVEN. PLEASE WRITE THE COMPLETE SOLUTIONS/DIAGRAMS LEGIBLY. NO LONG EXPLANATION NEEDED. USE 3 DECIMAL PLACES. BOX THE FINAL ANSWER. A pump discharges into a semi spherical tank at the bottom and cylindrical on top with a radius of 2 meters, and a total tank height of 8 meters. The flow rate is 80 gallons per minute and fluid specific gravity= 1.1. Determine the following: A) flow rate in kg/sec and time it takes to fill the tank in hours. B) the weight in tons of the liquid if full tank local gravity is 32.1 ft/sec squared. C) volume of the tank in metric gallons. D.) how many drums will be needed to transfer all of the tank contents (tank being full) to the drums?arrow_forwardI want the value of Cd from the graph and Results & Discussion for this ASAParrow_forward
- In NFPA diamond, red indicates the fire hazardEntropy is path functionAccording to hazard and risk assessment, if the occurrence is frequent and catastrophic, then it is unacceptableFor constant volume process, work is zeroA.) If all 4 statements are trueB.) If 3 of the 4 statements are trueC.) If 2 of the 4 statements are trueD.) If only 1 of the 4 statements is trueE.) If none of the 4 statements is truearrow_forwardQuestion 7 The following formulas are commonly used by engineers to predict the lift and drag of an airfoil: where L and Dare the lift and drag forces, V is the airspeed, S is the wing span, is the air density, and CL and CD are the lift and drag coefficients. Both CL and CD depend on α , the angle of attack, the angle between the relative air velocity and the airfoil’s chord line. Wind tunnel experiments for a particular airfoil have resulted in the following formulas. where α is in degrees.arrow_forwardPlease provide equations that you are using so I can follow along and learn how to do this and include conversions and units. In Jamestown, NY, a coal power plan consumes coal at a rate of 80 tons/hr. When coal burns, it generates approximately 30,000 kJ for every kg of coal burned. The cooling course for the plant is the nearby Chadakoin River nearby and, by code, the power plant cannot reject more than 13.19 x 109 MJ into the river annually. Determine the maximum average power output of this plant and it's maximum thermal efficiency. To check your work, enter the efficiency, as a decimal, in the box provided.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





