
(a)
Interpretation:
The major product obtained by the reaction of excess of given compound with
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
Chlorination:
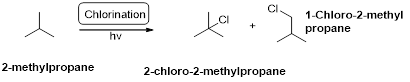
2-methylpropane undergoes radical chlorination and yields the 2-chloro-2-methylpropane and 1-chloro-2-methyl propane.
(b)
Interpretation:
The major product obtained by the reaction of excess of given compound with
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
Chlorination:
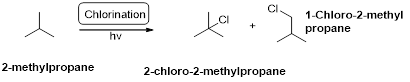
2-methylpropane undergoes radical chlorination and yields the 2-chloro-2-methylpropane and 1-chloro-2-methylpropane.
(c)
Interpretation:
The major product obtained by the reaction of excess of given compound with
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
Chlorination:
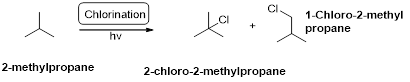
2-methylpropane undergoes radical chlorination and yields the 2-chloro-2-methylpropane and 1-chloro-2-methylpropane.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Organic Chemistry (8th Edition)
- Following are diastereomers (A) and (B) of 3-bromo-3,4-dimethylhexane. On treatment with sodium ethoxide in ethanol, each gives 3,4-dimethyl-3-hexene as the major product. One diastereomer gives the E alkene, and the other gives the Z alkene. Which diastereomer gives which alkene? Account for the stereoselectivity of each -elimination.arrow_forward-Ocimene is a pleasant-smelling hydrocarbon found in the leaves of certain herbs. It has the molecular formula C10H16 and a UV absorption maximum at 232 nm. On hydrogenation with a palladium catalyst, 2,6-dimethyloctane is obtained. Ozonolysis of -ocimene, followed by treatment with zinc and acetic acid, produces the following four fragments: (a) How many double bonds does -ocimene have? (b) Is -ocimene conjugated or nonconjugated? (c) Propose a structure for -ocimene. (d) Write the reactions, showing starting material and products.arrow_forwardAcid-catalyzed hydrolysis of the following epoxide gives a trans diol. Of the two possible trans diols, only one is formed. How do you account for this stereoselectivity?arrow_forward
- How many products are obtained following a monochlorination reaction (Cl2, UV) if the stereochemistry is not taken into consideration?arrow_forwardWhat is the major product formed from reacting cyclohexene in aqueous Br2, followed by treatment with potassium tert-butoxide?arrow_forwardCompound A has the formula C9H19Cl. B is a C9H19Br compound.A and B undergo base-promoted E2 elimination to give the same alkene C as the major product as well as different minor products.C reacts with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 2,6-dimethylheptane.Addition of HCl to C yields A as the major product.Propose structures for A and B.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

