
Concept explainers
(a)
Interpretation:
The major product obtained on treating the compounds in problem 29 with
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
Bromination:
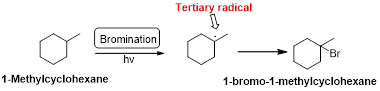
1-Methyl cyclohexane is undergoes radical bromination which forms 1-bromo-1-methyl cyclohexane therefore, bromination will occur where the tertiary radical is present. Because bromination will occur selectively in tertiary alkyl radical. (bromination reactions are more selective reaction)
(b)
Interpretation:
The major product obtained on treating the compounds in problem 29 with
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
Bromination:

1-Methylcyclohexane is undergoes radical bromination which forms 1-bromo-1-methylcyclohexane therefore, bromination will occur where the tertiary radical is present. Because bromination will occur selectively in tertiary alkyl radical. (bromination reactions are more selective reaction)
(c)
Interpretation:
The major product obtained on treating the compounds in problem 29 with
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
Bromination:
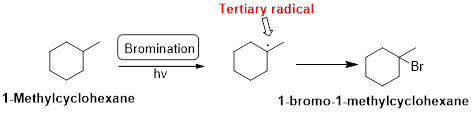
1-Methylcyclohexane is undergoes radical bromination which forms 1-bromo-1-methylcyclohexane therefore, bromination will occur where the tertiary radical is present. Because bromination will occur selectively in tertiary alkyl radical. (bromination reactions are more selective reaction)
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Organic Chemistry (8th Edition)
- Which structure shows the most stable intermediate formed when H—Br reacts with isobutylene (2-methylpropene)?arrow_forwardGiven the following reaction sequence: What are the reagents for reaction 7? A) HBr 2 eq B) Br2arrow_forwardThe following reaction has a ΔSsystem < 0 O2(g) → 2O(g) T or F can you explain why this is false?arrow_forward
- Why are SN1 reactions favored by polar protic solvents? Group of answer choices Polar protic solvents stabilize reaction intermediates Polar protic solvents cannot form hydrogen bonds. Polar protic solvents contribute leaving groups. Polar protic solvents contribute leaving groups.arrow_forwardwhat are the possible products from the SN1 reaction shown?arrow_forwardWhy does naphthalene undergo electrophilic substitution at C1 instead of C2? Could you draw a structure for the intermediate that helps explain this?arrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning




