
(a)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 43E
Electron dot structure and structural formula of
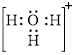
Explanation of Solution
In molecule
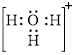
Figure 1

Figure 2
Solid line, in Figure 2, between the oxygen atom and the hydrogen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the atoms present in that bond.
An electron dot structure and structural formula of
(b)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 43E
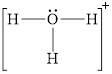
Electron dot structure and structural formula of
![]()
![]()
Explanation of Solution
In molecule
![]()
Figure 3
![]()
Figure 4
Solid line, in Figure 4, between the oxygen atom and the hydrogen atom shows the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the atoms present in that bond.
An electron dot structure and structural formula of
(c)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 43E
Electron dot structure and structural formula of
![]()
![]()
Explanation of Solution
In molecule
![]()
Figure 5
. ![]()
Figure 6
Solid line, in Figure 6, between the sulfur and hydrogen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the atoms present in that bond.
An electron dot structure and structural formula of
(d)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 43E
Electron dot structure and structural formula of
![]()
![]()
Explanation of Solution
In molecule
![]()
Figure 7
![]()
Figure 8
Each solid line, in Figure 8, between the carbon and nitrogen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the atoms present in that bond.
An electron dot structure and structural formula of
Want to see more full solutions like this?
Chapter 12 Solutions
Modified Mastering Chemistry with Pearson eText -- Standalone Access Card -- for Introductory Chemistry: Concepts and Critical Thinking (8th Edition)
- Which atoms can bond to sulfur so as to produce a positive partial charge on the sulfur atom?arrow_forwardDraw Lewis diagrams for the following ions. In the formula the symbol of the central atom is given first. (Hint:The valence octet may be expanded for the central atom.) (a) BrO4 (b) PCl6 (c) XeF6+arrow_forwardWould the following entries be correct when plugging into the ionic character formulae?arrow_forward
- Elements in the same group of the periodic table often formoxyanions with the same general formula. The anions arealso named in a similar fashion. Based on these observations,suggest a chemical formula or name, as appropriate, for eachof the following ions: (a) BrO4-, (b) SeO32-, (c) arsenate ion,(d) hydrogen tellurate ion.arrow_forwardGive a cation isoelectronic with fluoride ion______________________ 3.Arrange in increasingsize: sodiumion, nitride ion, magnesium ion, oxide ion, neon. (explain your rationale)______<______<______<_____<_____ Arrange in decreasingsize: sulfide ion, argon, potassium ion, calcium ion, chloride ion. (explain your rationale)______>______>______>_____>___arrow_forwarddisilicon pentaselenide, is it ionic or covalent?arrow_forward
- Write the chemical formula of the following. A. Calcium chloride B. Calcium chloratearrow_forwardHow many electrons will beryllium need to be removed in order for it to become a stable ion?arrow_forwardFor the Lewis electron dot formula for oxygen diiodide, OI2, there are ___ Regions of Electron Density around the O and the shape is ____arrow_forward
- Which of the following does not describe an ionic bond? A. Between a cation and an anion B. Dissolves into ions in solution C. An unequal exchange of electrons D. An equal sharing of electronsarrow_forwardExplain in terms of the distribution of charge, why H2O is considered a nonpolar compound.arrow_forwardwhich of the following isthe correct lewis structure of one phosphorous atom with the appropriate number of hydrogen atoms such that a neutral molecule is formed?arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning




