
Concept explainers
Interpretation:
The way in which Lewis structure of a molecule is helpful in determining its molecular shape is to be explained.
Concept introduction:
Lewis structure is a representation of a molecule which shows shared and unshared pair of electrons. It is helpful to determine the shape of a molecule.
Explanation of Solution
Lewis dot structure shows the bonding and non-bonding pair of electrons.
To determine the shape of molecule, the total number of bonding and non-bonding pairs is to be calculated for the central atom. Then these are arranged in a shape so that electron- electron repulsions are minimum.
For example: methane:
Its Lewis dot structure is:
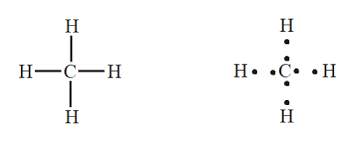
It shows carbon has 4 bonding pairs. Thus these pairs are to be arranged to minimize the electronic repulsion. So its shape is tetrahedral as:
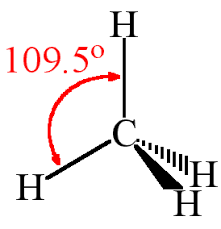
This way, molecular shape can be drawn with the help of Lewis structures.
Thus, Lewis structure of a molecule is helpful in determining its molecular shape
Chapter 12 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





