
Concept explainers
(a)
Interpretation: The Lewis structure and the molecular structure with polarity of molecule needs to be determined.
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that exist on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The hybridization and molecular structure of molecule determine the molecular structure of molecule.
For a molecule to be polar, it should have polar bond with no symmetry in the molecule.
(a)
Answer to Problem 109E
Explanation of Solution
In
Hence the best Lewis structure for
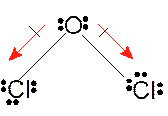
It is a polar molecule as it has two O-F polar bonds with bent geometry.
In
Hence the best Lewis structure for
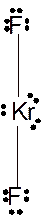
In
Hence the best Lewis structure for
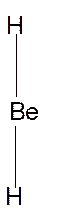
In
Hence the best Lewis structure for
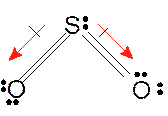
(c)
Interpretation: The Lewis structure and the molecular structure with polarity of molecule needs to be determined.
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that exist on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The hybridization and molecular structure of molecule determine the molecular structure of molecule.
For a molecule to be polar, it should have polar bond with no symmetry in the molecule.
(c)
Answer to Problem 109E
Explanation of Solution
In
Hence the best Lewis structure for
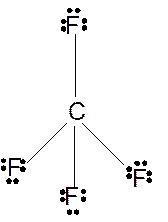
In
Hence the best Lewis structure for
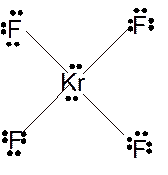
In
Hence the best Lewis structure for
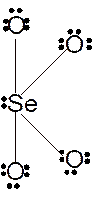
(d)
Interpretation: The Lewis structure and the molecular structure with polarity of molecule needs to be determined.
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that exist on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The hybridization and molecular structure of molecule determine the molecular structure of molecule.
For a molecule to be polar, it should have polar bond with no symmetry in the molecule.
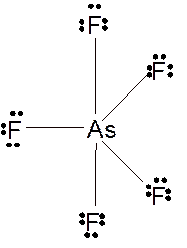
(d)
Answer to Problem 109E
Explanation of Solution
According to the given molecular formula, the structure of
Hence the best Lewis structure for
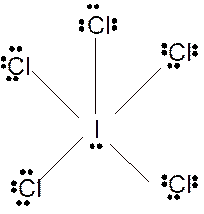
According to the given molecular formula, the structure of
Hence the best Lewis structure for
Want to see more full solutions like this?
Chapter 13 Solutions
Chemical Principles
- Write Lewis structures and predict the molecular structures of the following. (See Exercises 115 and 116.) a. OCl2, KrF2, BeH2, SO2 b. SO3, NF3, IF3 c. CF4, SeF4, KrF4 d. IF5, AsF5 Which of these compounds are polar?arrow_forwardPredict die molecular structure and bond angles for each molecule or ion in Exercises 88 and 94. a. POCl3, SO42, XeO4, PO43, ClO4 b. NF3, SO32, PO33, ClO3 c.ClO2, SCl2, PCl2 d. Considering your answers to parts a, b, and c. what conclusions can you draw concerning the structures of species containing the same number of atoms and the same number of valence electrons? (O3), sulfur dioxide, and sulfur trioxide.arrow_forwardThere are several molecular structures based on the trigonal bipyramid geometry (see Table 8.9). Three such structures are Which of the compounds in Exercises 91 and 92 have these molecular structures? PF5, SF4, ClF3 and Br3 SF6, ClF5, and XeF4arrow_forward
- An important observation supporting the concept of resonance in the localized electron model was that there are only three different structures of dichlorobenzene (C6H4C10). How does this fact support the concept of resonance (see Exercise 89)?arrow_forwardThe strucrure of TeF5 is Draw a complete Lewis structure for TeF5, and explain the distortion from the ideal square pyramidal structure. (See Exercise 26.)arrow_forwardPredict the molecular structure (including bond angles) for each of the following. (See Exercises 115 and 116.) a. XeCl2 b. ICl3 c. TeF4 d. PCl5arrow_forward
- Predict the molecular structure (including bond angles) for each of the following. (See Exercises 115 and 116.) a. ICl5 b. XeCl4 c. SeCl6arrow_forwardPredict the molecular structure and bond angles for each molecule or ion in Exercises 87 and 93. a. CCl4 b. NCl3 c. SeCl2 d. ICl a. NO2, NO3, N2O4 (N2O4 exists as O2NNO2.) b. OCN, SCN, N3 (Carbon is the central atom in OCN and SCN.)arrow_forwardThe most common exceptions to the octet rule are compounds or ions with central atoms having more than eight electrons around them. PF5, SF4, CIF3, and Br3 are examples of this type of exception. Draw the Lewis structure for these compounds or ions. Which elements, when they have to, can have more than eight electrons around them? How is this rationalized?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





