
Concept explainers
(a)
Interpretation: The Lewis structure for
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
Formal charge on each atom can be determined with the help of number of valence shell electrons, number of lone pair electrons and bond pair electrons. The formula for the formal charge can be written as:
(a)
Answer to Problem 86E
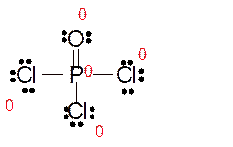
Explanation of Solution
Total number of valence electrons in
Hence the best Lewis structure for
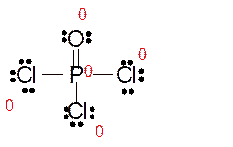
Here all bonded atoms have minimum formal charge therefore it must be the best Lewis structure.
(b)
Interpretation: The Lewis structure for
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
Formal charge on each atom can be determined with the help of number of valence shell electrons, number of lone pair electrons and bond pair electrons. The formula for the formal charge can be written as:
(b)
Answer to Problem 86E
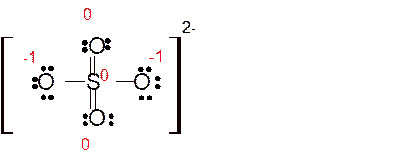
Explanation of Solution
Total number of valence electrons in
Hence the best Lewis structure for
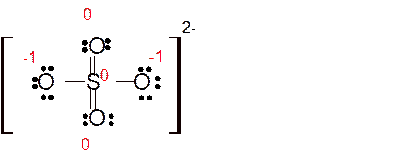
Here all bonded atoms have minimum formal charge therefore it must be the best Lewis structure.
(c)
Interpretation: The Lewis structure for
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
Formal charge on each atom can be determined with the help of number of valence shell electrons, number of lone pair electrons and bond pair electrons. The formula for the formal charge can be written as:
(c)
Answer to Problem 86E
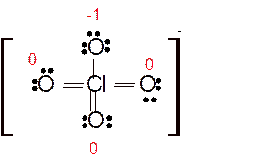
Explanation of Solution
Total number of valence electrons in
Hence the best Lewis structure for
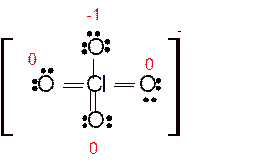
Here all bonded atoms have minimum formal charge therefore it must be the best Lewis structure.
(d)
Interpretation: The Lewis structure for
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
Formal charge on each atom can be determined with the help of number of valence shell electrons, number of lone pair electrons and bond pair electrons. The formula for the formal charge can be written as:
(d)
Answer to Problem 86E
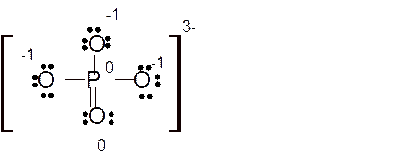
Explanation of Solution
Total number of valence electrons in
Hence the best Lewis structure for
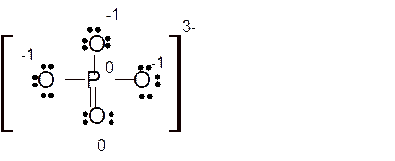
Here all bonded atoms have minimum formal charge therefore it must be the best Lewis structure.
(e)
Interpretation: The Lewis structure for
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
Formal charge on each atom can be determined with the help of number of valence shell electrons, number of lone pair electrons and bond pair electrons. The formula for the formal charge can be written as:
(e)
Answer to Problem 86E
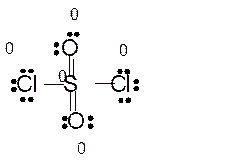
Explanation of Solution
Total number of valence electrons in
Hence the best Lewis structure for
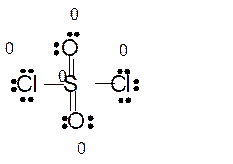
Here all bonded atoms have minimum formal charge therefore it must be the best Lewis structure.
(f)
Interpretation: The Lewis structure for
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
Formal charge on each atom can be determined with the help of number of valence shell electrons, number of lone pair electrons and bond pair electrons. The formula for the formal charge can be written as:
(f)
Answer to Problem 86E
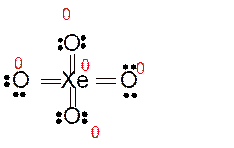
Explanation of Solution
Total number of valence electrons in
Hence the best Lewis structure for
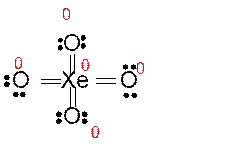
Here all bonded atoms have minimum formal charge therefore it must be the best Lewis structure.
(g)
Interpretation: The Lewis structure for
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
Formal charge on each atom can be determined with the help of number of valence shell electrons, number of lone pair electrons and bond pair electrons. The formula for the formal charge can be written as:
(g)
Answer to Problem 86E
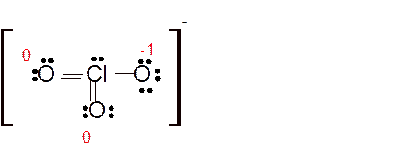
Explanation of Solution
Total number of valence electrons in
Hence the best Lewis structure for
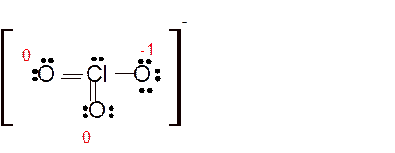
Here all bonded atoms have minimum formal charge therefore it must be the best Lewis structure.
(h)
Interpretation: The Lewis structure for
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
Formal charge on each atom can be determined with the help of number of valence shell electrons, number of lone pair electrons and bond pair electrons. The formula for the formal charge can be written as:
(h)
Answer to Problem 86E
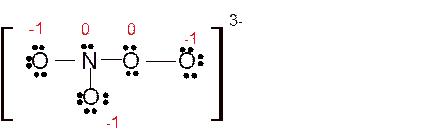
Explanation of Solution
Total number of valence electrons in
Hence the best Lewis structure for
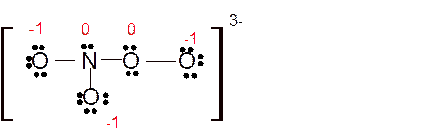
Here all bonded atoms have minimum formal charge therefore it must be the best Lewis structure.
Want to see more full solutions like this?
Chapter 13 Solutions
Chemical Principles
- (a) What is the physical basis for the VSEPR model?(b) When applying the VSEPR model, we count a double ortriple bond as a single electron domain. Why is this justified?arrow_forwardWhich of the molecules in Exercise 121 have net dipole moments (are polar)?arrow_forwardGive the expected hybridization of the central atom for the molecules or ions in Exercises 81 and 87 from Chapter 3.arrow_forward
- Derive Lewis structures for the compounds below. Furanarrow_forwardThe molecular ion S3N3 has the cyclic structure All SN bonds are equivalent. (a) Give six equivalent resonance hybrid Lewis diagrams for this molecular ion. (b) Compute the formal charges on all atoms in the molecular ion in each of the six Lewis diagrams. (c) Determine the charge on each atom in the polyatomic ion, assuming that the true distribution of electrons is the average of the six Lewis diagrams arrived at in parts (a) and (b). (d) An advanced calculation suggests that the actual charge resident on each N atom is 0.375 and on each S atom is +0.041 . Show that this result is consistent with the overall +1 charge on the molecular ion.arrow_forward• describe chemical bonding using a model based on the overlap of atomic orbitals and recognize sonic of the limitations of this simple model.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





