
(a)
Interpretation:
The rate constant for the growth in the number of transistor on an integrated circuit has to be determined using the given plot ln N versus year.
Concept introduction:
Rate of the reaction is the change in the concentration of reactant or a product with time.
The rate law expresses the relationship of the
Rate equation for the general reaction
Order of a reaction: The sum of exponents of the concentrations in the rate law for the reaction is said to be order of a reaction.
For first order reaction,
Moore’s law states that the number of transistors per square inch on integrated circuits had doubled every year since their invention (1958).
(a)
Explanation of Solution
Given plot of ln N versus t (year) is shown below,
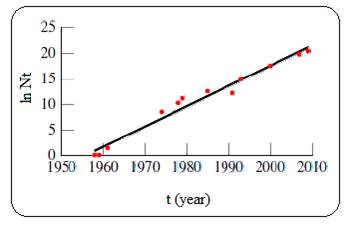
Figure 1
The plot of ln N versus t is linear for a process which follows first order kinetics. And so the given process follows first order kinetics.
The rate can be described using the equation,
Where N is the number of transistor on an integrated circuit, which is roughly doubles every 1.5 year according to the Moore’s law.
For first order reaction,
For this case, the equation can be rearranged as follows,
Comparing this equation to the straight line equation (
(b)
Interpretation:
The time required for
Concept introduction:
The rate law expresses the relationship of the rate of a reaction to the rate constant.
Rate equation for the general reaction
Order of a reaction: The sum of exponents of the concentrations in the rate law for the reaction is said to be order of a reaction.
For first order reaction,
Moore’s law states that the number of transistors per square inch on integrated circuits had doubled every year since their invention (1958).
(b)
Explanation of Solution
Given plot of ln N versus t (year) is shown below,

Figure 1
The time required for
For first order reaction,
This value is very close to the value mentioned in Moore’s law.
(c)
Interpretation:
The number of transistors on an integrated circuit
Concept introduction:
The rate law expresses the relationship of the rate of a reaction to the rate constant.
Rate equation for the general reaction
Order of a reaction: The sum of exponents of the concentrations in the rate law for the reaction is said to be order of a reaction.
For first order reaction,
Moore’s law states that the number of transistors per square inch on integrated circuits had doubled every year since their invention (1958).
(c)
Explanation of Solution
Given plot of ln N versus t (year) is shown below,

Figure 1
The time required for
For first order reaction,
For this case, the equation can be rearranged as follows,
Assume the year 1960 as
The year 2100 would corresponds to
Substituting known values in the above mentioned equation,
Thus, there will be
Want to see more full solutions like this?
Chapter 13 Solutions
Chemistry
- Amoxicillin is an antibiotic packaged as a powder. When it is used to treat babies and small animals, the pharmacist or veterinarian must suspend it in water, so that it can be administered orally with a medicine dropper. The label says to dispose of unused suspension after 14 days. It also points out that refrigeration is required. In the context of this chapter, what is implied in the latter two statements?arrow_forwardSilicon forms a series of compounds analogous to the al-kanes and having the general formula SinH2n+2. The first of these compounds is silane, SiH4, which is used in the electronics industry to produce thin ultrapure silicon films. SiH4(g) is somewhat difficult to work with because it is py-ropboric at room temperature—meaning that it bursts into flame spontaneously when exposed to air. (a) Write an equation for the combustion of SiH4(g). (The reaction is analogous to hydrocarbon combustion, and SiO2 is a solid under standard conditions. Assume the water produced will be a gas.) (b) Use the data from Appendix E to calculate ? for this reaction. (c) Calculate G and show that the reaction is spontaneous at 25°C. (d) Compare G for this reaction to the combustion of methane. (See the previous problem.) Are the reactions in these two exercises enthalpy or entropy driven? Explain.arrow_forwardSubstances that poison a catalyst pose a major concern for many engineering designs, including those for catalytic converters. One design option is to add materials that react with potential poisons before they reach the catalyst. Among the commonly encountered catalyst poisons are silicon and phosphorus, which typically form phosphate or silicate ions in the oxidizing environment of an engine. Group 2 elements are added to the catalyst to react with these contaminants before they reach the working portion of the catalytic converter. If estimates show that a catalytic converter will be exposed to 625 g of silicon during its lifetime, what mass of beryllium would need to be included in the design?arrow_forward
- Define stability from both a kinetic and thermodynamic perspective. Give examples to show the differences in these concepts.arrow_forwardUse the kinetic theory to justify the following observations: (a) the rate of a reaction in the gas phase depends on the energy with which two molecules collide, which in turn depends on their speeds; (b) in the Earth’s atmosphere, light gases, such as H2 and He, are rare but heavier gases, such as O2, CO2, and N2, are abundant.arrow_forwardThe highest building in Montreal is the ‘1 square building’, with a height of 203m.The atmospheric pressure in the streets of Montreal is P1 = 9.9 x 104Pa.Suppose air density is constant : ρair= 1,2 kg/m3 and g = 9.8 m/s2. Suppose the rooftop temperature to be T = 298K, and the air composition is 100%N2 molecules. The collision cross-section of N2 molecules is 0.43 nm2.*f. Find the collision frequency. Is it in the order of magnitude that you wereexpecting?arrow_forward
- Determine the average rate of change of BB from ?=0 s�=0 s to ?=392 s.�=392 s. A⟶2BA⟶2B Time (s) Concentration of A (M) 0 0.7300.730 196196 0.4450.445 392392 0.160arrow_forwardCO(g)+2H2(g)⇄CH3OH(g)K=2.2×104at298K A stoichiometric mixture of CO(g) and H2(g) was allowed to react in two different 2.0L rigid containers at a constant temperature of 298K. The reaction is represented by the equation above. Diagram 1 represents the uncatalyzed reaction and diagram 2 represents the catalyzed reaction one hour after the reactants were mixed. Which of the following correctly explains the experimental results represented in the particle diagrams? A Although the reaction is thermodynamically favorable because ΔG°<0 based on the value of K, only the catalyzed reaction could proceed in one hour because its reactant molecules had a higher average kinetic energy. B Although the reaction is thermodynamically favorable because ΔG°<0 based on the value of K, only the catalyzed reaction could proceed in one hour because it has a lower activation-energy reaction pathway. C The reaction is not thermodynamically favorable because ΔG°>0 based on the value of K, but…arrow_forward1) Sulfuryl chloride, SO2Cl2, is a highly reactive gaseous compound. When heated, it decomposes as follows: SO2Cl2(g) ® SO2(g) + Cl2(g). This decomposition is endothermic. A sample of 3.509 grams of SO2Cl2 is placed in an evacuated 1.00 liter bulb and the temperature is raised to 375K. (a) What would be the pressure in atmospheres in the bulb if no dissociation of the SO2Cl2(g) occurred? (b) When the system has come to equilibrium at 375K, the total pressure in the bulb is found to be 1.43 atmospheres. Calculate the partial pressures of SO2, Cl2, and SO2Cl2 at equilibrium at 375K. (c) Give the expression for the equilibrium constant (either Kp or Kc) for the decomposition of SO2Cl2(g) at 375K. Calculate the value of the equilibrium constant you have given, and specify its units. (d) If the temperature were raised to 500K, what effect would this have on the equilibrium constant? Explain briefly. Note: Please answer just C and D. Thank you.arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





