
(a)
Interpretation:
The given synthesis scheme is to be converted to word form that can be used as instructions in the laboratory.
Concept introduction:
The synthesis scheme is the balanced chemical equation written for carrying out a sequence of reactions with specified steps. The synthetic step displays that the reactants are converted to the product by reacting with reagents in the required conditions.
The structures on the left side of the reaction arrow (![]() ) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on the right side of the reaction arrow
) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on the right side of the reaction arrow
(![]() ) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the
) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the
Answer to Problem 13.28P
The word form for the given synthesis scheme is:
The starting ![]() . Then, treat
. Then, treat ![]() with phosphoric acid at
with phosphoric acid at ![]() to form
to form ![]() .
.
Explanation of Solution
The given synthesis scheme is:
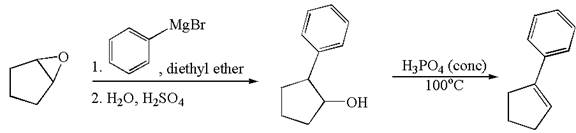
In the given synthetic route, the reactant having functional group epoxide is converted to an alcohol named ![]() which is further converted to an
which is further converted to an ![]() on reaction with appropriate reagents as mentioned. In the first step, the first reagent used is phenylmagnesium bromide in the solvent diethyl ether, and the second reagent is
on reaction with appropriate reagents as mentioned. In the first step, the first reagent used is phenylmagnesium bromide in the solvent diethyl ether, and the second reagent is ![]() which represents the aqueous acidic condition. In the second step, the reagent is phosphoric acid and
which represents the aqueous acidic condition. In the second step, the reagent is phosphoric acid and ![]() is the reaction temperature. Thus, the word form of the above synthetic scheme can be written as follows:
is the reaction temperature. Thus, the word form of the above synthetic scheme can be written as follows:
The starting epoxide reacts with phenylmagnesium bromide in the solvent diethyl ether followed by aqueous acid, to form ![]() . Then, treat
. Then, treat ![]() with phosphoric acid at
with phosphoric acid at ![]() to form
to form ![]() .
.
The given synthesis scheme is converted to word form by identifying the names of reactants, reagents, and products.
(b)
Interpretation:
The given synthesis scheme is to be converted to word form that can be used as instructions in the laboratory.
Concept introduction:
The synthesis scheme is the balanced chemical equation written for carrying out a sequence of reactions with specified steps. The synthetic step displays that the reactants are converted to products by reacting with the reagents in the required conditions.
The structures on the left side of the reaction arrow (![]() ) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on right side of the reaction arrow (
) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on right side of the reaction arrow (![]() ) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the functional groups involved in the reactants and the functional groups produced in the product are to be identified.
) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the functional groups involved in the reactants and the functional groups produced in the product are to be identified.
Answer to Problem 13.28P
The word form for the given synthesis scheme is:
Phenylehtanone reacts with lithium diisopropylamide followed by iodomethane, to produce phenylpropanone. Then, add the molecular bromine in the presence of acetic acid to form ![]() which further reacts with sodium acetate to yield the final product.
which further reacts with sodium acetate to yield the final product.
Explanation of Solution
The given synthesis scheme is:
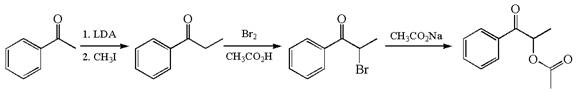
The given synthetic route is of three steps. In the first step, the reactant having functional group ![]() alpha bromo ketone named
alpha bromo ketone named ![]() , which, in the third step, is further converted to the final product having the ester functional group. In the first step, the first reagent used is lithium diisopropylamide, and the second reagent is iodomethane. In the second step, the reagent is molecular bromine in acetic acid. The reagent for the third step is sodium acetate. Thus, the word form of the above synthetic scheme can be written as follows:
, which, in the third step, is further converted to the final product having the ester functional group. In the first step, the first reagent used is lithium diisopropylamide, and the second reagent is iodomethane. In the second step, the reagent is molecular bromine in acetic acid. The reagent for the third step is sodium acetate. Thus, the word form of the above synthetic scheme can be written as follows:
Phenylehtanone reacts with lithium diisopropylamide followed by iodomethane to produce phenylpropanone. Add the molecular bromine in presence of acetic acid to phenylpropanone to form ![]() which further reacts with sodium acetate to yield THE final product.
which further reacts with sodium acetate to yield THE final product.
The given synthesis scheme was converted to word form by identifying the names of reactants, reagents, and products.
(c)
Interpretation:
The given synthesis scheme is to be converted to word form that can be used as instructions in the laboratory.
Concept introduction:
The synthesis scheme is the balanced chemical equation written for carrying out a sequence of reactions with specified steps. The synthetic step displays that the reactants are converted to the product by reacting with reagents in the required conditions.
The structures on the left side of the reaction arrow (![]() ) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on right side of the reaction arrow (
) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on right side of the reaction arrow (![]() ) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the functional groups involved in the reactants, and the functional groups produced in the product are to be identified.
) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the functional groups involved in the reactants, and the functional groups produced in the product are to be identified.
Answer to Problem 13.28P
The word form for the given synthesis scheme is:
Heat ![]() in aqueous hydrochloric acid to form
in aqueous hydrochloric acid to form ![]() , add phosphorous tribromide to it to produce
, add phosphorous tribromide to it to produce ![]() . Then, react
. Then, react ![]() with sodium azide to yield the final product.
with sodium azide to yield the final product.
Explanation of Solution
The given synthesis scheme is:
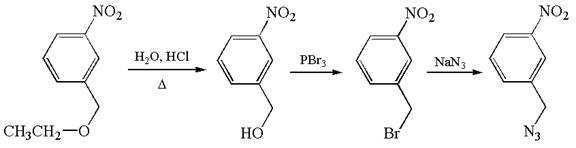
The given synthetic route is of three steps. The nitro functional group remains as it is throughout the reaction sequence, and so, is not considered. In the first step, ether functional group is converted to an alcohol. In the second step, ![]() group of alcohol is replaced by bromine, which in the third step is replaced by
group of alcohol is replaced by bromine, which in the third step is replaced by ![]() . In the first step, the reagent used is
. In the first step, the reagent used is ![]() that represents acidic condition where
that represents acidic condition where ![]() is the symbol used for heat. In the second step, the reagent is
is the symbol used for heat. In the second step, the reagent is ![]() , phosphorous tribromide, and in the third step, the reagent used is sodium azide,
, phosphorous tribromide, and in the third step, the reagent used is sodium azide, ![]() . Thus, the word form of the above synthetic scheme can be written as follows:
. Thus, the word form of the above synthetic scheme can be written as follows:
Heat ![]() in aqueous hydrochloric acid to form
in aqueous hydrochloric acid to form ![]() , and add phosphorous tribromide to it to produce
, and add phosphorous tribromide to it to produce ![]() . Then, react
. Then, react ![]() with sodium azide to yield the final product.
with sodium azide to yield the final product.
The given synthesis scheme was converted to word form by identifying the names of reactants, reagents, and products.
Want to see more full solutions like this?
Chapter 13 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- The SN2 reaction can be carried out intramolecularly (in the same molecule). What product do you expect to form when treating 4-Bromo-1-butanol with a base?justify the reasonarrow_forwardDetermine a synthesis for the following molecule using organic reagents no larger than 2 or 3 carbons or benzene. You may use any inorganic reagents necessary. For each reaction cite a reference that shows the use of this type of reaction.arrow_forwardCome up with a synthetic route to achieve the following transformation.arrow_forward
- Several reagents and several organic structures are shown below. Construct a multistep synthetic route for the synthesis of acetylene from ethane by dragging the appropriate pieces into the bins. Note that each bin will hold only one item, and not every given reagent or structure will be used. (Stoichiometry is omitted.)arrow_forwardfrom the list provided below, place the best reagent into each of the five reactions shown in order to complete the given synthetic conversion.arrow_forwardList the appropriate reagents that can be used in places with question marks in the following series of reactions.arrow_forward
- Enolates are formed by deprotonation of an α-carbon hydrogen. Answer the following questions about enolate formation. In the molecule shown, select the α-carbon hydrogen that would be removed to form an enolate when NaOEt is used as a base. Draw the thermodynamic enolate that results for the molecule in Part 1. Draw only the enolate resonance form that includes a formal charge on the α carbon. Be sure to indicate that formal charge as well as any lone pair of electrons in your answer.arrow_forwardWe now continue the use of organic chemistry reaction roadmaps. Because of the unique nature of the new reactions presented, we recommend that you make a new roadmap only for Chapters 2023. To make your own roadmap for Chapters 2023, take a blank sheet of paper and write the following functional groups in the orientations shown. Fill the entire sheet of paper and leave plenty of room between functional groups. Most students find it helpful to use a poster-sized sheet of paper filled out in landscape orientation. We now continue the use of organic chemistry reaction roadmaps. Because of the unique nature of the new reactions presented, we recommend that you make a new roadmap only for Chapters 2023. To make your own roadmap for Chapters 2023, take a blank sheet of paper and write the following functional groups in the orientations shown. Fill the entire sheet of paper and leave plenty of room between functional groups. Most students find it helpful to use a poster-sized sheet of paper filled out in landscape orientation.arrow_forwardFill in the missing reagents and chemical structures in the following synthetic proposal.arrow_forward
- what is a multistep synthesis for this transformation. add any reagents or compounds to help accomplish itarrow_forwardSecondary alcohols are often dehydrated in an E2 reaction to give an alkene. Elimination follows Zaitsev's rule to give the more substituted alkene as the major product. Since the reaction occurs via an E2 mechanism, there is no risk of rearrangement of the carbon skeleton as could possibly occur if the elimination occurred via an E1 mechanism with a carbocation intermediate.Draw curved arrows to show the movement of electrons in this step of the mechanism.arrow_forwardDraw the alkyl bromide(s) you would use in a malonic ester synthesis of ethyl cyclopentanecarboxylate. You do not have to consider stereochemistry. If no reaction occurs, draw ethyl cyclopentanecarboxylate. If more than one alkyl bromide is needed, draw them all. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate structures with + signs from the drop-down menu.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

