
(a)
Interpretation:
Sulfur belongs to group of the periodic table, along with . The element has an
Sulfur has three allotropes: Rhombic, Monoclinic and amorphous.
Sulfur is a non- metal which exists in different crystal structure known as allotropes. The stable form at room temperature is called rhombic sulfur and when this is heated slowly above about it converts into monoclinic sulfur.
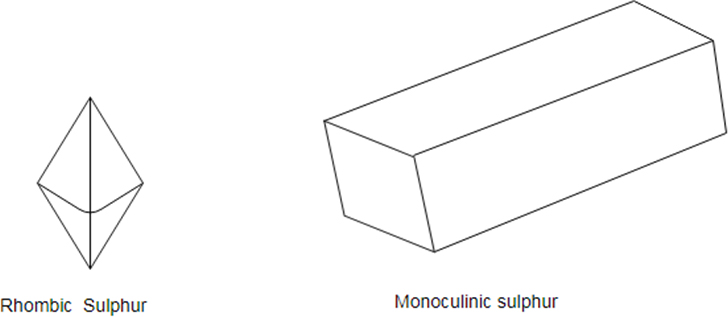
Only difference between the rhombic and monoclinic types is the arrangement of the molecules in space.
Concept introduction:
Phase diagram represent change in state of substance with change in pressure and temperature.
There are some common line between solid state, liquid state and gaseous state. The line are known as sublimation curve common line between solid state and gaseous state condensation curve (common region between liquid and gas) and meeting curve (common region between solid and liquid state)
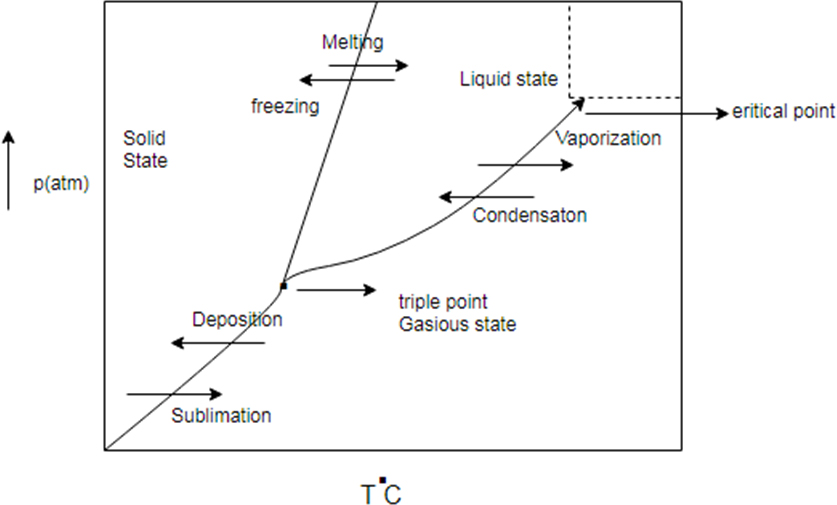
Generic phase diagram and conversion between states
To determine:
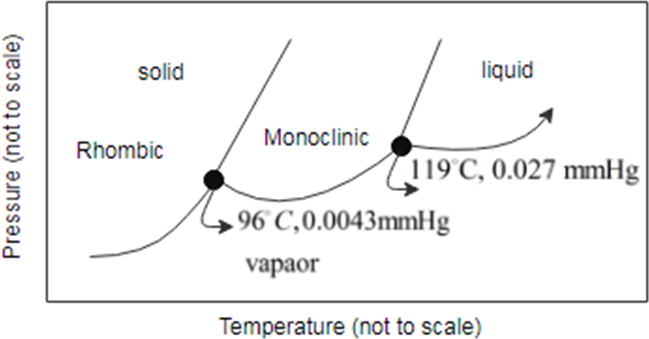
Consider the phase diagram for sulfur shown here. The rhombic and monoclinic are two solid with different structures.
a. The pressure below which solid sulfur sublimes
(b)
Interpretation:
Sulfur belongs to group of the periodic table, along with . The element has an atomic number of and atomic mass of , four oxidation states
Sulfur has three allotropes: Rhombic, Monoclinic and amorphous (no shape plastic).
Sulfur is a non- metal which exists is different crystal structure known as allotropes. The stable form at room temperature is called rhombic sulfur and when this is heated slowly above about it converts into monoclinic sulfur.
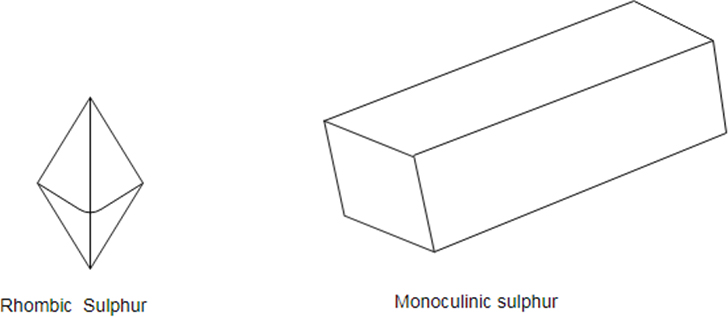
Only difference between the rhombic and monoclinic types is the arrangement of the molecules in space.
Concept Interdiction:
Phase diagram represents change in state of substance with change in pressure and temperature.
There are some common line between solid state, liquid state and gaseous state. The line are known as sublimation curve common line between solid state and gaseous state condensation curve (common region between liquid and gas) and melting curve (common region between solid and liquid state)
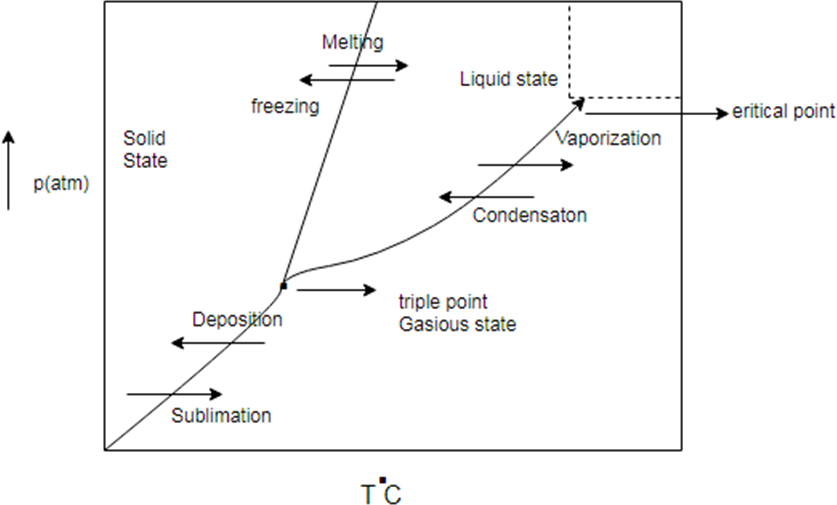
Generic phase diagram and conversion between states
To determine: between the two solid states of sulfur which is denser
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Study Guide For Chemistry: Structure And Properties
- The enthalpy of vaporization of water is larger than its enthalpy of fusion. Explain why.arrow_forwardExplain why the enthalpies of vaporization of the following substances increase in the order CH4NH3H2O, even though all three substances have approximately the same molar mass.arrow_forwardWhy do liquids have a vapor pressure? Do all liquids have vapor pressures? Explain. Do solids exhibit vapor pressure? Explain. How does vapor pressure change with changing temperature? Explain.arrow_forward
- A special vessel (see Fig. 10.45) contains ice and supercooled water (both at 10C) connected by vapor space. Describe what happens to the amounts of ice and water as time passes.arrow_forwardExplain why evaporation leads to cooling of the liquid.arrow_forwardIf you've ever opened a bottle of rubbing alcohol or other solvent on a warm day, you may have heard a little “whoosh” as the vapor that had built up above the liquid escapes. Describe on a microscopic basis how a vapor pressure builds up in a closed container above a liquid. What processes in the container give rise to this phenomenon?arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





