
Treating either 1-chloro-3-methyl-2-butene or 3-chloro-3-methyl-1-butene with
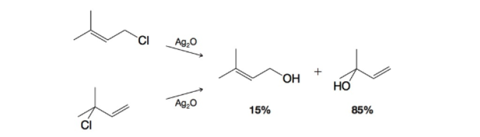
(a) Write a mechanism that accounts for the formation of these products.
(b) What might explain the relative proportions of the two
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Organic Chemistry, 12e Study Guide/Student Solutions Manual
Additional Science Textbook Solutions
Organic Chemistry
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Chemistry: The Central Science (13th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Chemistry: A Molecular Approach (4th Edition)
Organic Chemistry (9th Edition)
- Draw a structural formula of an alkene that undergoes acid-catalyzed hydration to give each alcohol as the major product (more than one alkene may give each alcohol as the major product). (a) 3-Hexanol (b) 1-Methylcyclobutanol (c) 2-Methyl-2-butanol (d) 2-Propanolarrow_forward(b) 3-methyl-2-butanol reacts with concentrated sulphuric acid to form 2-methyl-2- butene. Write the mechanism for the reaction.arrow_forwardGive the structure of the product formed when each of the following alkenes reacts with bromine in water: (a) 2-Methyl-1-butene (c) 3-Methyl-1-butene (b) 2-Methyl-2-butene (d) 1-Methylcyclopentenearrow_forward
- (1) Predict the outcome of the addition of HBr to (a) trans-2-pentene, (b) 2-methyl-2-butene, and (c) 4-methylcyclohexene. How many isomers can be formed in each case? (2) Addition of HBr to 3,3-dimethyl-1-butene gives a mixture of two isomeric alkyl bromide products. Draw structures for the two products, and give a mechanistic explanation for their formation.arrow_forwardPhotochemical chlorination of 2,2,4-trimethylpentane gives four isomeric monochlorides. (a) Write structural formulas for these four isomers. (b) The two primary chlorides make up 65% of the monochloride fraction. Assuming that all the primary hydrogens in 2,2,4-trimethylpentane are equally reactive, estimate the percentage of each of the two primary chlorides in the product mixture.arrow_forwardDisiamylborane adds only once to alkynes by virtue of its two bulky secondary isoamylgroups. Disiamylborane is prepared by the reaction of BH3 # THF with an alkene.(a) Draw the structural formulas of the reagents and the products in the preparation ofdisiamylboranearrow_forward
- Write the bond line formula of the following compounds: (a) 4-methyl-2-hexene, two geometrical (stereoisomers) isomers (b) 3-fluoro-2-methylheptanol (3-fluoro-2-methylheptan-1-ol) (c) 4-methyl-hex-1-yn-3-olarrow_forwardCompounds B and C are hydrocarbons with the structural formulae as shown below. CH, „CH, в (a) Name compounds B and C according to the IUPAC nomenclature. (b) Both B and C can undergo oxidation reaction with the same oxidizing agent. Write chemical equations involved and explain the differences between these two reactions. (c) Name one reaction that converts B to methylcyclohexane.arrow_forwardPropose a structural formula for the product(s) when each of the following alkenes is treated with H2O/H2SO4. Why are two products formed in part (b), but only one in parts (a) and (c)? (a) 1-Hexene gives one alcohol with a molecular for- mula of C6H14O. (b) 2-Hexene gives two alcohols, each with a molecu- lar formula of C6H14O. (c) 3-Hexene gives one alcohol with a molecular for- mula of C6H14O.arrow_forward
- (b) (1-chloromethyl)cyclopentane, C6H11CI reacts with aqueous sodium hydroxide, NaOH to produce a primary alcohol AA. When CsH11Cl is added with magnesium, Mg in ether, an organometallic compound BB is formed. When compound BB reacts with ethanal, CH3CHO, a secondary alcohol cC is formed. The molecular structure of C6H11CI is given below. (1-klorometil)siklopentana, C6H11CI bertindak balas dengan akues natrium hidroksida, NaOH bagi menghasilkan satu alkohol primer AA. Apabila C6H11CI ditambah dengan magnesium, Mg dalam eter, sebatian organologam BB terbentuk. Apabila sebatian BB bertindak balas dengan etanal, CH3CHO, satu alkohol sekunder CC dihasilkan. Struktur molekul bagi C6H11CI diberikan di bawah. (1-chloromethyl)cyclopentanearrow_forwardWrite structural formulas for compounds that meet the following descriptions:(a) An alkene, C6H12, that cannot have cis–trans isomersand whose longest chain is 5 carbons long(b) An alkene with a chemical formula of C10H12 that hascis–trans isomers and contains a benzene ring.arrow_forward2-chloropropane is a major product of the reaction of chlorine with propane under ultraviolet light. Write the mechanism for this reaction including the initiation step and the two propagation steps.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





