
Organic Chemistry, 12e Study Guide/Student Solutions Manual
12th Edition
ISBN: 9781119077329
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 4PP
Practice Problem 13.4
From each set of resonance structures that follow, designate the one that would contribute most to the hybrid and explain your choice:
(a)
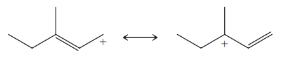
(b)
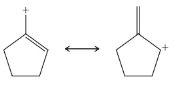
(c)
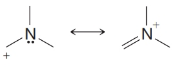
(d)
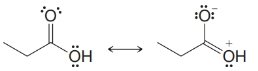
(e)

(f)

Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Provide a structure for the compound C,HN, using the given information.
IR: 3281 cm-1
'H NMR: 8 1.1 (8H, t, J = 7 Hz), 8 2.66 (4H, q, J = 7 Hz), 8 2.83 (4H, s). (Hint: The triplet at 8 1.1 conceals another broad
resonance that contributes to the integral.)
Draw the structure for C,HN,.
16
2
12.6 (opq) Predict the major product and give the name of the reaction
(b) From each pair below identify the relatively more acidic compound, giving clear
reasons in each case for your choice.
(i)
(iii)
and
CH3COCH2Cl
1,3-Cyclohexandione and
CH₂Br
and
CH3COCHC12
1,4-Cyclohexandione
H
CH₂Br
Chapter 13 Solutions
Organic Chemistry, 12e Study Guide/Student Solutions Manual
Ch. 13 - Prob. 1PPCh. 13 - Prob. 2PPCh. 13 - Prob. 3PPCh. 13 - Practice Problem 13.4 From each set of resonance...Ch. 13 - Practice Problem 13.5 The following enol (an...Ch. 13 - Prob. 6PPCh. 13 - Practice Problem 13.7
Two compounds, A and B, have...Ch. 13 - Prob. 8PPCh. 13 - Prob. 9PPCh. 13 - Prob. 10PP
Ch. 13 - Prob. 11PPCh. 13 - Prob. 12PPCh. 13 - Prob. 13PPCh. 13 - Prob. 14PPCh. 13 - Prob. 15PPCh. 13 - Practice Problem 13.16
Diels–Alder reactions also...Ch. 13 - Prob. 17PPCh. 13 - Prob. 18PCh. 13 - What product would you expect from the following...Ch. 13 - Prob. 20PCh. 13 - Prob. 21PCh. 13 - Provide the reagents necessary for each of the...Ch. 13 - Prob. 23PCh. 13 - Prob. 24PCh. 13 - Prob. 25PCh. 13 - When 1-pentene reacts with N-bromosuccinimide...Ch. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 29PCh. 13 - Prob. 30PCh. 13 - 13.31 Provide a mechanism that explains formation...Ch. 13 - 13.32 Provide a mechanism that explains formation...Ch. 13 - Treating either 1-chloro-3-methyl-2-butene or...Ch. 13 - Prob. 34PCh. 13 - Prob. 35PCh. 13 - Although both 1-bromobutane and 4-bromo-1-butene...Ch. 13 - Prob. 37PCh. 13 - Prob. 38PCh. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Prob. 42PCh. 13 - Prob. 43PCh. 13 - 13.44 When furan and maleimide undergo a...Ch. 13 - Two controversial hard insecticides are aldrin and...Ch. 13 - Prob. 46PCh. 13 - Prob. 47PCh. 13 - Prob. 48PCh. 13 - Prob. 49PCh. 13 - Prob. 50PCh. 13 - Explain the product distribution below based on...Ch. 13 - Mixing furan (Problem 13.44) with maleic anhydride...Ch. 13 - Prob. 53PCh. 13 - Prob. 54PCh. 13 - Prob. 1LGPCh. 13 - Prob. 2LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
A KNO3 solution containing 45 g of KNO3 per 100 g of water is cooled from 40Cto0C. What happens during cooling?...
Introductory Chemistry (6th Edition)
4. 38 Strontium has four naturally occurring isotopes, with mass numbers 84, 86, 87, arid 88.
a. Write the atom...
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
11.1 Why are the intermolecular attractive forces stronger in liquids and solids than they are in gases?
Chemistry: The Molecular Nature of Matter
Sketch the following spectra that would be obtained for 2-chloroethanol: a. The 1H NMR spectrum for an anhydrou...
Organic Chemistry
Joints of high quality can be formed by friction welding. Consider the friction welding of two 40-mm-diameter I...
Fundamentals of Heat and Mass Transfer
[14.110] The following mechanism has been proposed for the gas-phase reaction of chloroform (CHCI3) and chlorin...
Chemistry: The Central Science (13th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- PRACTICE PROBLEM 8.20 Specify the alkene and reagents needed to synthesize each of the following diols. OH HO- (a) (b) (c) HO. н он HO (racemic) (racemic)arrow_forwardPractice Problem 13.36b Problem 13.34 outlines a general method for the preparation of cis- or trans-disubstituted epoxides. Using that method, identify what reagents you would use to prepare the following epoxide from acetylene: H" 'Et The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagent(s) in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A B C D DMP or PCC На, Pt EtBr PHCH2BR E F H H2SO4, H20, HgSO4 МСРВА (RCO3зН) Na, NH3 (/) H2, Lindlar's cat. I K TSCI, py PhBr NANH2 1) EtMgBr; 2) H30+arrow_forwardUsing hex-1-ene as your starting material, show how you would synthesize the following compounds. (Once you haveshown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.)(a) 1,2-dibromohexanearrow_forward
- Using cyclooctyne as your starting material, show how you would synthesize the following compounds. (Once you haveshown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.)(a) cis-cyclooctene (b) cyclooctane (c) trans-1,2-dibromocyclooctanearrow_forwardAnswer ALL parts of this question. The structure shown below is that of one of the geometric isomers (the E- isomer) of the antidepressant, Doxepin: (a) (b) CH₂CH₂N(CH3)2 H E-Doxepin Explain how the Cahn Ingold Prelog sequence rules can be used to rank groups in order of priority, using the four groups attached to the C=C double bond in Doxepin as illustrations. Based on the order of priority of the four groups determined in part (a) of this question, explain why the isomer of Doxepin shown above is defined as the E-isomer. (c) In general terms, explain why this isomer might have different effects in the human body from its geometric isomer.arrow_forwardWhich of the following compounds are aromatic and anti-aromatic? Give justifications for your answer. i) ii) iii) +, iv)arrow_forward
- Based on the structures shown, which isomer should exhibit the highest aromatic stabilization? (a) (c) (d) య్నవ (0)arrow_forwardA compound of formula C6H10O2 shows only two absorptions in the proton NMR: a singlet at 2.67 ppm and a singlet at2.15 ppm. These absorptions have areas in the ratio 2:3. The IR spectrum shows a strong absorption at 1708 cm-1. Proposea structure for this compound.arrow_forwardFor each of the following structures, would you predict these molecules to possess aromaticity? Why or why not? (a) (c) (d) (a) 4 (b) 6 (c) 6 iπ electrons, antiaromatic iT electrons in each ring, aromatic TT electrons, aromatic (d) 00 8 TT electrons, antiaromaticarrow_forward
- Using cyclooctyne as your starting material, show how you would synthesize the following compounds. (Once you haveshown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.)(a) cis-cyclooctenearrow_forwardUsing cyclooctyne as your starting material, show how you would synthesize the following compounds. (Once you haveshown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.)(a) cis-cyclooctene (b) cyclooctane (c) trans-1,2-dibromocyclooctane(d) cyclooctanone (e) 1,1-dibromocyclooctane (f) 3-bromocyclooctene(g) cyclooctane-1,2-dionearrow_forwardDiscuss the hybridization, aromaticity, and stability of the following organic intermediate. Also, arrange them in the increasing order of stability. CHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY