
(a)
Interpretation:
The product for the given reaction has to be identified.
Concept introduction:
The commonly used Peroxy acids are meta-Chloroperoxybenzoic acid (MCPBA) and Peroxyacetic acid. This process is stereospecific.
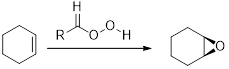
Reaction of Grignard reagent with epoxide will form alcohols. Grignard reagent will add to the less substituted carbon. The
(b)
Interpretation:
The product for the given reaction has to be identified.
Concept introduction:
Alkoxymercuration-demercuration: the process where alcohols can be prepared from alkene, results in the markovnikov’s addition of
NaBH4 (Sodium borohydride):
Sodium borohydride is used as a reducing agent.
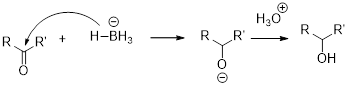
(c)
Interpretation:
The product for the given reaction has to be identified.
Concept introduction:
Epoxides from Peroxy acids:
The commonly used Peroxy acids are meta-Chloroperoxybenzoic acid (MCPBA) and Peroxyacetic acid. This process is stereospecific.
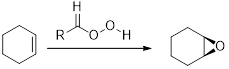
Base catalyzed ring opening of epoxide:
The nucleophile will attack at the less substituted position under basic conditions
(d)
Interpretation:
The product for the given reaction has to be identified.
Concept introduction:
Reaction of metal with alcohol:
Metals can react with alcohol to produce alkoxide ion. For an example, ethanol can be react with sodium to produce hydrogen gas and sodium ethoxide.
(e)
Interpretation:
The product for the given reaction has to be identified.
Concept introduction:
Reaction of metal with alcohol:
Metals can react with alcohol to produce alkoxide ion. For an example, ethanol can be react with sodium to produce hydrogen gas and sodium ethoxide.
Epoxides from Peroxy acids:
The commonly used Peroxy acids are meta-Chloroperoxybenzoic acid (MCPBA) and Peroxyacetic acid. This process is stereospecific.
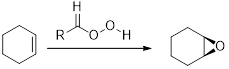
Acid-catalyzed ring-opening of epoxide: The epoxide ring is protonated and the nucleophile attack depends on the electronic or steric effect (nature of epoxide).
Regiochemistry: when the epoxide is unsymmetrical, the nucleophile attack at the more substituted position of the protonated epoxide ring.
Stereochemistry: when the nucleophile attack takes place at chiral center, an inversion of configuration is obtained.
Base catalyzed ring opening of epoxide:
The nucleophile will attack at the less substituted position under basic conditions
(f)
Interpretation:
The product for the given reaction has to be identified.
Concept introduction:
Reaction of Grignard reagent with epoxide will form alcohols. Grignard reagent will add to the less substituted carbon. The
The reactions of Epoxides with strong nucleophiles requires a strong driving force that helps in the removal of ring strain associated with the three-membered ring of an Epoxide. These ring opening reactions also occur under acidic conditions.
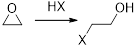
The mechanism of acid-catalyzed ring opening of an Epoxide occurs in two steps.
In first step, the protonation of Epoxide occurs.
In second step, the attack of nucleophile occurs in the protonated Epoxide by
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Organic Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





