
Concept explainers
(a)
Interpretation:
The equation for the reaction of interhalogen
Concept Introduction:
Interhalogen compounds:
The compounds which are only composed of two different halogens are known as Interhalogen.
Disproportionation
The formation of products of oxidized and reduced substances from a simultaneous redox reaction of single reactant is known as disproportionation redox reaction.
(a)
Explanation of Solution
Given depiction is,
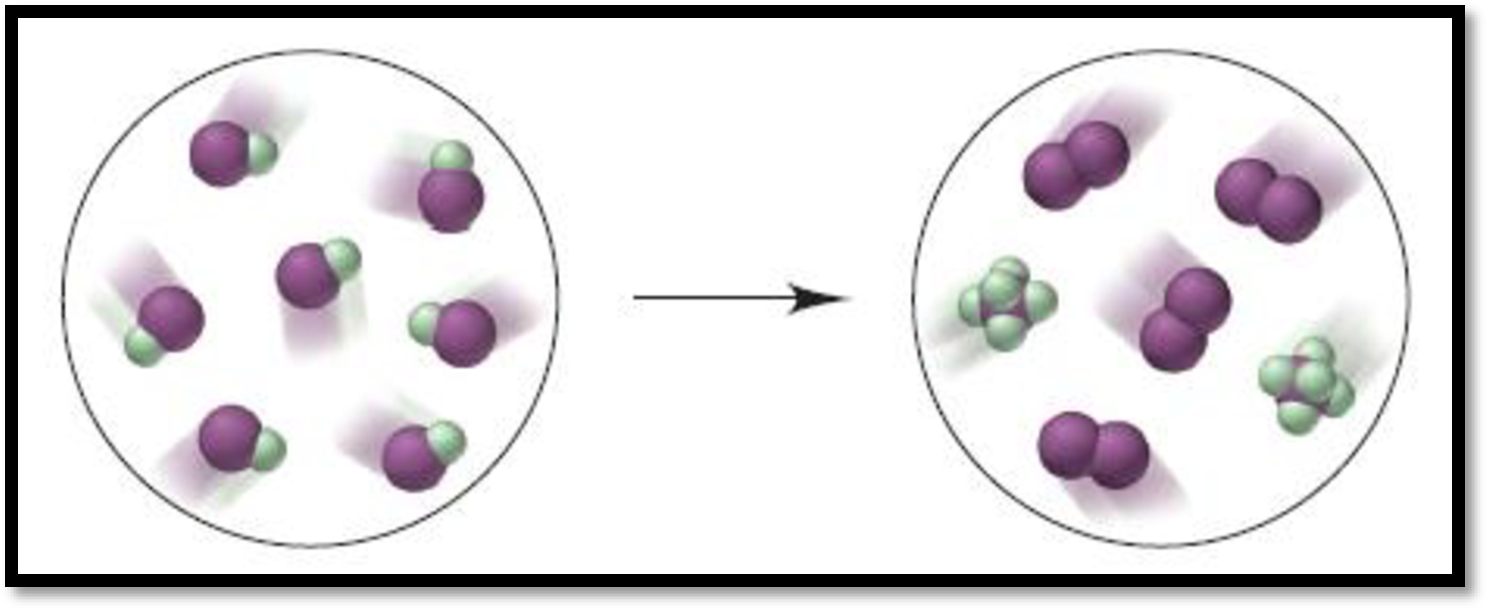
Figure 1
From the given depiction, brown colour = iodine and green coloured are Fluorine atoms.
Therefore, the depiction refers the formation of
Hence, the balanced equation for the give reaction is,
(b)
Interpretation:
Name of the product of given redox reaction of
Concept Introduction:
Disproportionation redox reaction:
The formation of products of oxidized and reduced substances from a simultaneous redox reaction of single reactant is known as disproportionation redox reaction.
Interhalogen compounds:
The compounds which are only composed of two different halogens are known as Interhalogen.
(b)
Explanation of Solution
Given depiction is,
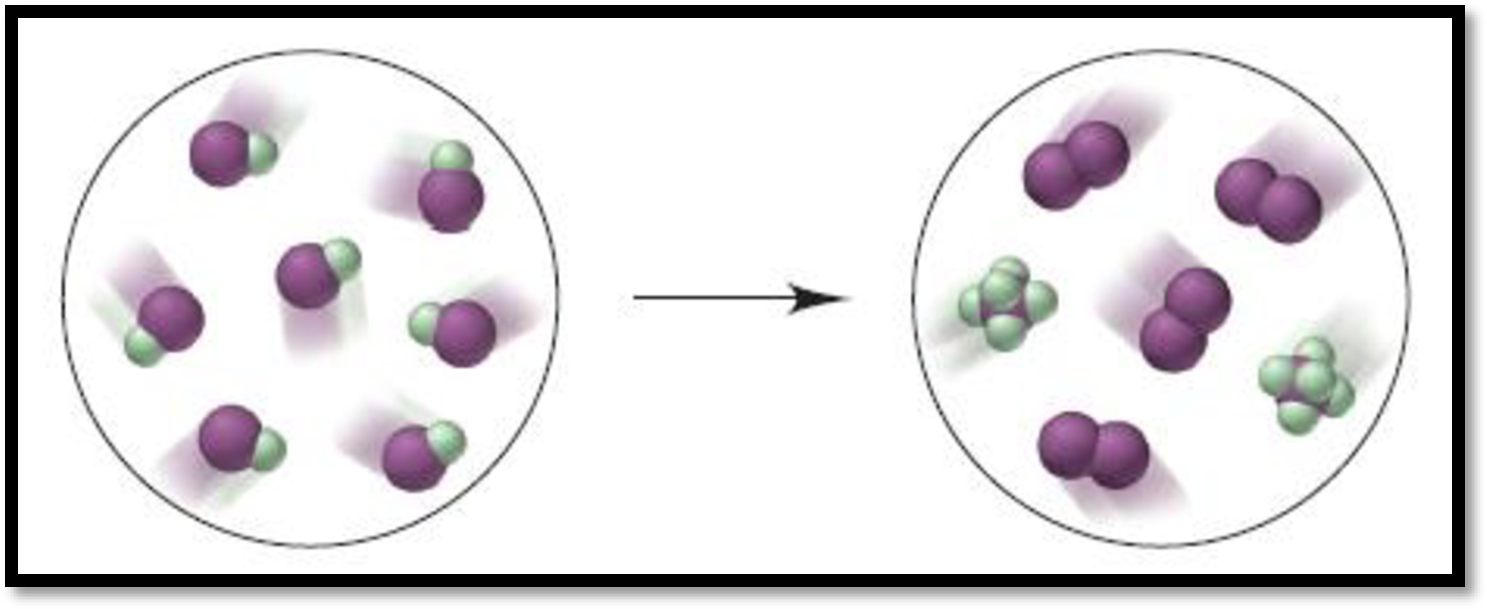
Figure 1
From the given depiction, brown colour = iodine and green coloured are Fluorine atoms.
Therefore, the depiction refers the formation of
The balanced equation for the give reaction is,
Product of above reaction is Iodine penta-fluoride.
(c)
Interpretation:
Name of given redox reaction of
Concept Introduction:
Disproportionation redox reaction:
The formation of products of oxidized and reduced substances from a simultaneous redox reaction of single reactant is known as disproportionation redox reaction.
Interhalogen compounds:
The compounds which are only composed of two different halogens are known as Interhalogen.
(c)
Explanation of Solution
Given depiction is,
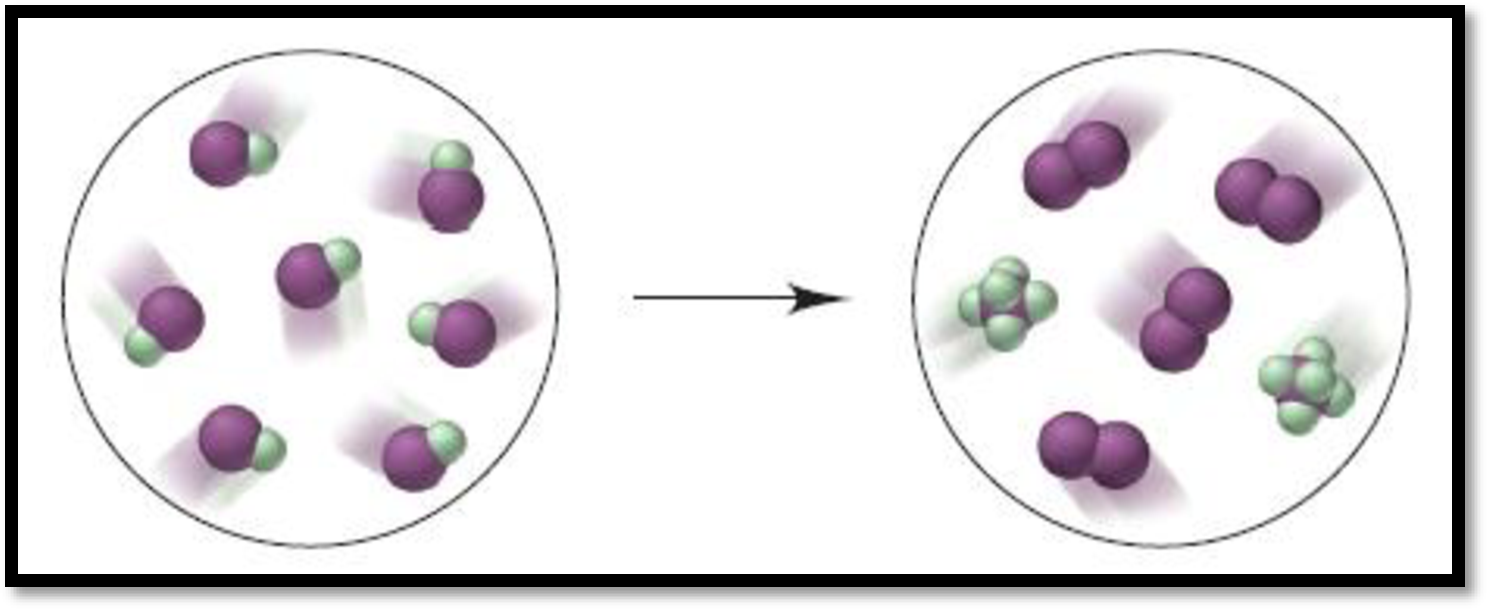
Figure 1
From the given depiction, brown colour = iodine and green coloured are Fluorine atoms.
Therefore, the depiction refers the formation of
The balanced equation for the give reaction is,
Therefore, the above reaction shows given reaction is disproportionation reaction.
(d)
Interpretation:
Mass of product that formed from
Concept Introduction:
Disproportionation redox reaction:
The formation of products of oxidized and reduced substances from a simultaneous redox reaction of single reactant is known as disproportionation redox reaction.
Interhalogen compounds:
The compounds which are only composed of two different halogens are known as Interhalogen.
Mole:
Mole of substance that present in taken mole of sample is calculated by mass of substance is divided by molar mass of the substance.
(d)
Explanation of Solution
Given depiction is,
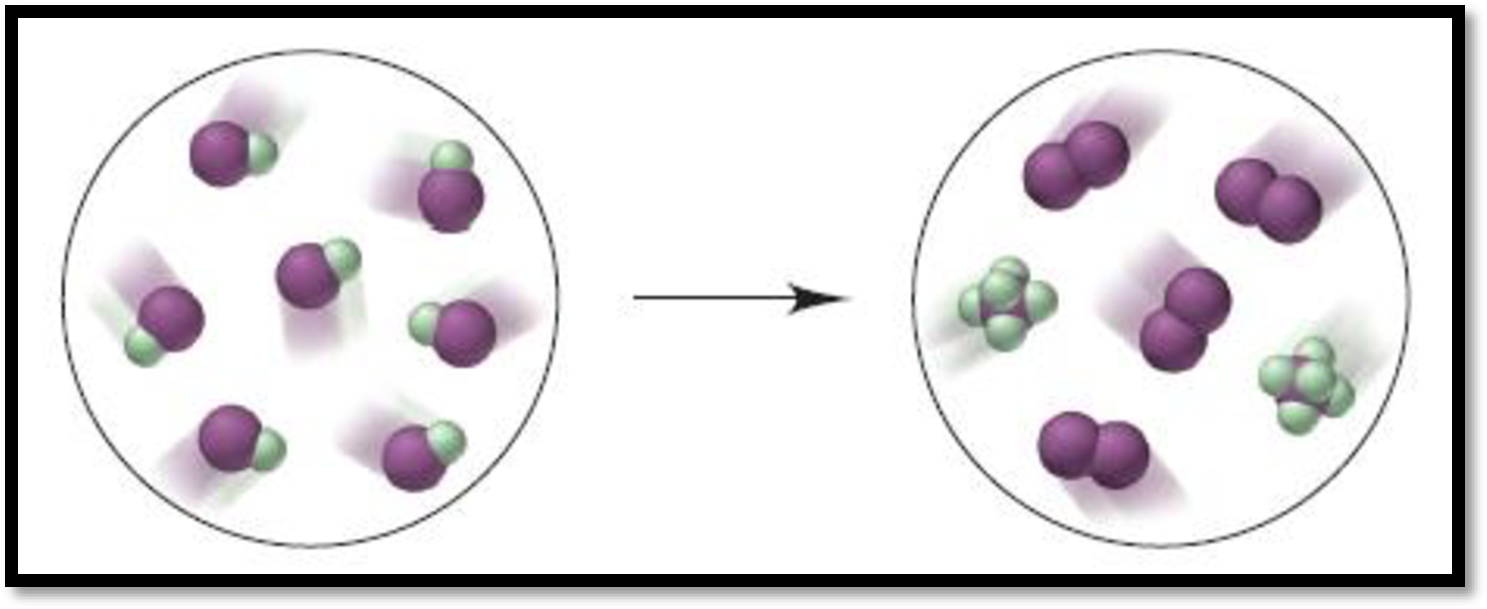
Figure 1
From the given depiction, brown = iodine and green = Fluorine atoms.
Therefore, the depiction refers the formation of
The balanced equation for the give reaction is,
Therefore, the above reaction shows given reaction is disproportionation reaction.
Mass of
Molar mass of
Mass of
Molar mass of
Hence,
Want to see more full solutions like this?
Chapter 14 Solutions
Chemistry: The Molecular Nature of Matter and Change
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





