
Fundamentals of General, Organic and Biological Chemistry Volume 1, 5th custom edition for Spokane Community College
1st Edition
ISBN: 9781323562789
Author: John McMurry, David Ballantine, Carl Hoeger
Publisher: Pearson Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 14, Problem 14.28AP
Interpretation Introduction
Interpretation:
The reason why the alcohols have more boiling point than the ether should be explained.
Concept Introduction:
Alcohol:
The compound containing hydroxyl
Example is given below
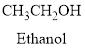
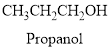
Ether:
The aliphatic or
Example is given below
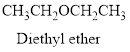
Hydrogen bond:
The electrostatic attraction between a hydrogen atom and more electronegative atom such as nitrogen (
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What structural feature is necessary for an alcohol to undergo oxidation reactions?
Which do you think has a higher boiling point, pentane or neopentane (2,2-dimethylpropane)? Why?
If the dehydration reaction of an alcohol is successful, what changes would be seen in the IR spectrum for the product compared to the starting material
Chapter 14 Solutions
Fundamentals of General, Organic and Biological Chemistry Volume 1, 5th custom edition for Spokane Community College
Ch. 14.1 - Identify each of the following compounds as an...Ch. 14.1 - Prob. 14.2PCh. 14.2 - Prob. 14.3PCh. 14.2 - Prob. 14.4PCh. 14.2 - Prob. 14.5PCh. 14.3 - Prob. 14.6PCh. 14.3 - For each of the following molecules, (i) redraw...Ch. 14.4 - Prob. 14.1MRPCh. 14.4 - Provide the mechanism for the dehydration of...Ch. 14.4 - Prob. 14.3MRP
Ch. 14.4 - Prob. 14.8PCh. 14.4 - What alcohols yield the following alkenes as the...Ch. 14.4 - Prob. 14.10KCPCh. 14.4 - What products would you expect from oxidation of...Ch. 14.4 - Prob. 14.12PCh. 14.4 - Prob. 14.13KCPCh. 14.5 - Prob. 14.14PCh. 14.5 - Prob. 14.15PCh. 14.7 - Prob. 14.1CIAPCh. 14.7 - Prob. 14.2CIAPCh. 14.7 - Prob. 14.3CIAPCh. 14.7 - Prob. 14.16PCh. 14.8 - What disulfides would you obtain from oxidation of...Ch. 14.9 - Prob. 14.18PCh. 14.10 - Prob. 14.19PCh. 14.10 - Prob. 14.20PCh. 14.10 - Prob. 14.4CIAPCh. 14.10 - Prob. 14.5CIAPCh. 14.10 - Prob. 14.6CIAPCh. 14.10 - Prob. 14.7CIAPCh. 14 - Prob. 14.21UKCCh. 14 - Prob. 14.22UKCCh. 14 - Prob. 14.23UKCCh. 14 - Prob. 14.24UKCCh. 14 - Prob. 14.25UKCCh. 14 - How do alcohols, ethers, and phenols differ...Ch. 14 - What is the structural difference between primary,...Ch. 14 - Prob. 14.28APCh. 14 - Prob. 14.29APCh. 14 - The Taxane nucleus is shown here; it is the basis...Ch. 14 - Vitamin E has the structure shown. Identify the...Ch. 14 - Give systematic names for the following alcohols:...Ch. 14 - Give systematic names for the following compound...Ch. 14 - Draw structures corresponding to the following...Ch. 14 - Draw structures corresponding to the following...Ch. 14 - Prob. 14.36APCh. 14 - Locate the alcohol functional groups in the taxane...Ch. 14 - Prob. 14.38APCh. 14 - Prob. 14.39APCh. 14 - Prob. 14.40APCh. 14 - Prob. 14.41APCh. 14 - Prob. 14.42APCh. 14 - Prob. 14.43APCh. 14 - Assume that you have samples of the following two...Ch. 14 - Which of the following alcohols can undergo...Ch. 14 - The following alkenes can be prepared by...Ch. 14 - Prob. 14.47APCh. 14 - Prob. 14.48APCh. 14 - What alcohols would you oxidize to obtain the...Ch. 14 - Prob. 14.50APCh. 14 - What is the structural relationship between a...Ch. 14 - Prob. 14.52APCh. 14 - Prob. 14.53APCh. 14 - Prob. 14.54APCh. 14 - Prob. 14.55APCh. 14 - Prob. 14.56APCh. 14 - Prob. 14.57APCh. 14 - Identify the chiral center(s) in each of the...Ch. 14 - Are the following molecules chiral or achiral? If...Ch. 14 - Prob. 14.60CPCh. 14 - Prob. 14.61CPCh. 14 - 1-Propanol is freely soluble in water, 1-butanol...Ch. 14 - Prob. 14.63CPCh. 14 - Prob. 14.64CPCh. 14 - Prob. 14.65CPCh. 14 - Prob. 14.66CPCh. 14 - Prob. 14.67CPCh. 14 - Prob. 14.68CPCh. 14 - Prob. 14.69CPCh. 14 - Prob. 14.70CPCh. 14 - Prob. 14.71CPCh. 14 - Prob. 14.72CPCh. 14 - (a)Draw all possible cyclic C7H14O alcohol isomers...Ch. 14 - Prob. 14.74GPCh. 14 - Prob. 14.75GP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Why do you think compounds containing the heavy metal lead are poisonous?arrow_forwardExplain why the hydride ion, H -, has a noble gas configuration.arrow_forwardConsider the following acids and their ionization constant, determine which conjugate base is HCOOH Ka = 1.7 x 10-4 (b) HCN Ka = 4.9 x 10-10arrow_forward
- How can you tell in an IR spectrum if the compound has just one or two oxygens? What is the difference between them? Thank you!arrow_forwardWhat is the mass in grams of 24.2 moles of NaCl?arrow_forwardCompare the densities of low-density polyethylene (LDPE) and high-density poly- ethylene (HDPE) with the densities of the liquid alkanes.How might you account for the differences between them?arrow_forward
- Identify the functional groups in the following compoundsarrow_forwardEmily added so much anhydrous na2so4 to dichloromethane solution of caffeine that no liquid can be seen anymore .how can she fix this mistake and recover the caffeine?arrow_forwardWhat is the empirical formula for a compound that is 26.56% potassium, 35.41% chromium, and 38.03% oxygen?arrow_forward
- The liquids butan-1-ol and butanal have similar molar masses. Which is expected to have the higher boiling point? Explain your choices.arrow_forwardIf one compound has the formula C5H10 and another has the formula C4H10, are the two compounds isomers? Explain.arrow_forwardWhat prefixes are used in naming the following?(a) A 1,3-disubstituted benzene(b) A 1,4-disubstituted benzenearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Biodiversity hotspots and functional diversity; Author: Stockholm Resilience Centre TV;https://www.youtube.com/watch?v=Gr_eIsFOKr4;License: Standard Youtube License