
Concept explainers
(a)
Interpretation:
The carbonyl containing product should be identified when the given alcohol undergoes oxidation reaction.
Concept introduction:
Oxidation reaction:
Alcohol undergoes oxidation reaction using oxidising agent like
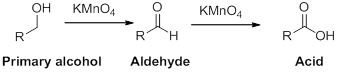
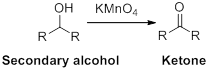
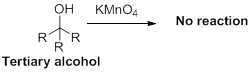
(b)
Interpretation:
The carbonyl containing product should be identified when the given alcohol undergoes oxidation reaction.
Concept introduction:
Oxidation reaction:
Alcohol undergoes oxidation reaction using oxidising agent like
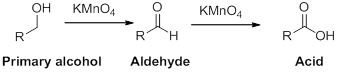
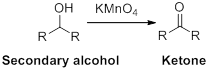
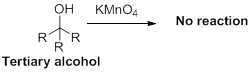
(c)
Interpretation:
The product should be identified when it is undergoes oxidation reaction.
Concept introduction:
Oxidation reaction:
Alcohol undergoes oxidation reaction using oxidising agent like
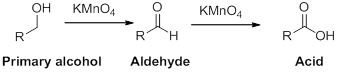
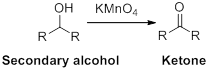
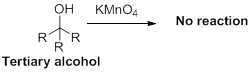
(d)
Interpretation:
The carbonyl containing product should be identified when the given alcohol undergoes oxidation reaction.
Concept introduction:
Oxidation reaction:
Alcohol undergoes oxidation reaction using oxidising agent like
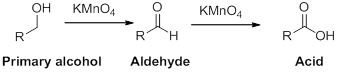
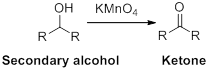
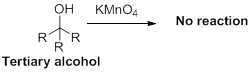
(e)
Interpretation:
The carbonyl containing product should be identified when the given alcohol undergoes oxidation reaction.
Concept introduction:
Oxidation reaction:
Alcohol undergoes oxidation reaction using oxidising agent like
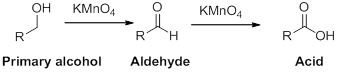
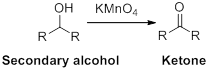
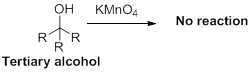
(b)
Interpretation:
The carbonyl containing product should be identified when the given alcohol undergoes oxidation reaction.
Concept introduction:
Oxidation reaction:
Alcohol undergoes oxidation reaction using oxidising agent like
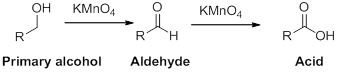
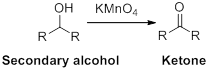
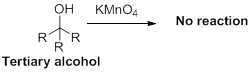
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Fundamentals of General, Organic and Biological Chemistry Volume 1, 5th custom edition for Spokane Community College
- Under standard conditions, will the following reaction proceed spontaneously as written?arrow_forward1-34. Indicate whether each of the following statements about enantiomers is true or false. (a to d)arrow_forwardDraw a Fischer projection formula for the enantiomer of each of the following monosaccharides. (a to d)arrow_forward
- With respect to the parent compound, explain in detail how modifying the rings shown as A, B, C, D and E in morphine can affect the biological activity associated with the moleculearrow_forwardThe explosive trinitrotoluene (TNT) is made by carrying out three successive nitration reactions on toluene. If these nitrations only occur in the ortho and para positions relative to the methyl group, what is the structure of TNT?arrow_forwardWhat functional groups are present in the peaks?arrow_forward
- Draw condensed structural formulas for all products obtained from the complete hydrolysis of the following triacylglycerol.arrow_forwardGive the full biochemical name for a D-aldopentose with the following pattern of chiral centers: carbon #2 = D, carbon #3 = D.arrow_forwardFor A, B, C, D, E, F, identify the circled functional groups and linkages in the compound in the picture.arrow_forward
- Draw the structure of the osazone formed when Beta-D-Xylofuranose is added with 2,4-dinitrophenylhydrazinearrow_forwardThe reaction of methoxy benzene with hydrogen iodide will yield a phenol and an alkyl halide. Which of following choices is the correct combination of the products?arrow_forwardIs D-2-deoxygalactose the same chemical as D-2-deoxyglucose? Explain.arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





