
Concept explainers
(a)
Interpretation:
State True or false.
The two most important reactions of alcohols are their acid-catalyzed dehydration to give
Concept Introduction:
A catalyst is the molecule which is used to speed up the chemical reaction, not being consumed within the procedure. Acids are used as catalysts are needed for an acid catalyzed hydration.
A dehydration reaction is aeration when any organic substance loses the water molecule to form an alkene.
Answer to Problem 14.29P
True.
Explanation of Solution
Alcohols are organic compounds containing -OH group. It undergoes in the presence of acid to form an alkene. Dehydration is the removal of water from alcohol.
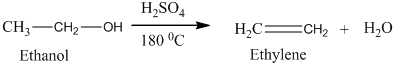
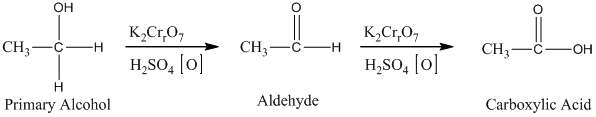
Alcohols undergo oxidation to yield aldehyde, ketone, and carboxylic acid. The primary alcohol in an acid catalyzed oxidation gives an aldehyde and further acid catalyzed oxidation gives a corresponding acid.
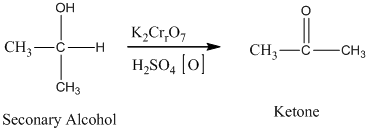
The secondary alcohol in an acid catalyzed oxidation fives a ketone.
Therefore, this statement is True.
(b)
Interpretation:
State True or false.
The acidity of alcohols is comparable to that of water.
Concept Introduction:
Chemical reaction is the procedure to transform the chemical substance in the new substance. During this procedure sometimes, acid is involved which is the substance which could either accept electron pairs or donate protons in the reactions.
A catalyst is the molecule which is used to speed up the chemical reaction, not being consumed within the procedure. Acids are used as catalysts are needed for an acid catalyzed hydration.
A dehydration reaction is aeration when any organic substance loses the water molecule to form an alkene.
Answer to Problem 14.29P
True.
Explanation of Solution
The strength of an acid can be measured by the acid dissociation constant Ka while the pKa value is logarithmic value of the acid dissociation constant. So, the alcohol has the nearly same pKa value as of water.
Therefore, this statement is True.
(c)
Interpretation:
State True or false.
Water-insoluble alcohols and water-insoluble phenols react with string bases to give water soluble salts.
Concept Introduction:
Chemical reaction is the procedure to transform the chemical substance in the new substance. During this procedure sometimes, acid is involved which is the substance which could either accept electron pairs or donate protons in the reactions.
A catalyst is the molecule which is used to speed up the chemical reaction, not being consumed within the procedure. Acids are used as catalysts are needed for an acid catalyzed hydration.
A dehydration reaction is aeration when any organic substance loses the water molecule to form an alkene.
Answer to Problem 14.29P
False.
Explanation of Solution
Alcohol is a water-soluble compound. It is soluble in water because of the hydrogen bonding between oxygen and hydrogen atoms of water and alcohol molecules. Phenol is also water soluble because of hydrogen bonding. Water-soluble alcohol and water-insoluble phenol react with string bases to form water-soluble salt.
Therefore, this statement is False.
(d)
Interpretation:
State True or false.
Acid catalyzed dehydration of cyclohexanol gives cyclohexene.
Concept Introduction:
Chemical reaction is the procedure to transform the chemical substance in the new substance. During this procedure sometimes, acid is involved which is the substance which could either accept electron pairs or donate protons in the reactions.
A catalyst is the molecule which is used to speed up the chemical reaction, not being consumed within the procedure. Acids are used as catalysts are needed for an acid catalyzed hydration.
A dehydration reaction is aeration when any organic substance loses the water molecule to form an alkene.
Answer to Problem 14.29P
False.
Explanation of Solution
Acid-catalyzed dehydration of cyclohexanol gives corresponding alkene. In the presence of sulfuric acid, cyclohexanol gives cyclohexene.
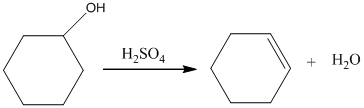
Therefore, this statement is False.
(e)
Interpretation:
State True or false.
When the acid-catalyzed dehydration of an alkene can yield isomeric alkenes, the alkene with the greater number of hydrogens in the carbons of the double bond generally predominates.
Concept Introduction:
Chemical reaction is the procedure to transform the chemical substance in the new substance. During this procedure sometimes, acid is involved which is the substance which could either accept electron pairs or donate protons in the reactions.
A catalyst is the molecule which is used to speed up the chemical reaction, not being consumed within the procedure. Acids are used as catalysts are needed for an acid catalyzed hydration.
A dehydration reaction is aeration when any organic substance loses the water molecule to form an alkene.
Answer to Problem 14.29P
False.
Explanation of Solution
Acid-catalyzed dehydration of an alcohol gives an isomeric alkene. The isomeric alkene, which has a lower number of hydrogen atoms in the double bond, has a grater yield. Hence, when butanol undergoes an acid-catalyzed dehydration, 2-butene is the major product.
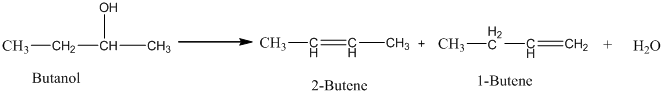
Therefore, this statement is False.
(f)
Interpretation:
State True or false.
The acid-catalyzed dehydration of 2-butanol gives predominantly 1-butene.
Concept Introduction:
Chemical reaction is the procedure to transform the chemical substance in the new substance. During this procedure sometimes, acid is involved which is the substance which could either accept electron pairs or donate protons in the reactions.
A catalyst is the molecule which is used to speed up the chemical reaction, not being consumed within the procedure. Acids are used as catalysts are needed for an acid catalyzed hydration.
A dehydration reaction is aeration when any organic substance loses the water molecule to form an alkene.
Answer to Problem 14.29P
False.
Explanation of Solution
An acid catalyzed dehydration of butanol gives predominantly 2-butene. The yield of 1-butene is approximately because in the acid-catalyzed dehydration reaction of an alcohol, the major product has the lower number of hydrogen atoms attached to the carbon atoms with double bonds. Thus, 2-Butene is the major product.
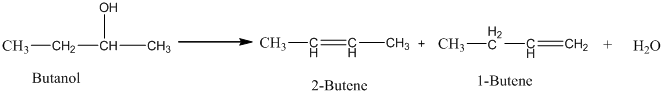
Therefore, this statement is False.
(g)
Interpretation:
State True or false.
The oxidation of a primary alcohol gives either an aldehyde or a carboxylic acid depending on experimental conditions.
Concept Introduction:
Chemical reaction is the procedure to transform the chemical substance in the new substance. During this procedure sometimes, acid is involved which is the substance which could either accept electron pairs or donate protons in the reactions.
A catalyst is the molecule which is used to speed up the chemical reaction, not being consumed within the procedure. Acids are used as catalysts are needed for an acid catalyzed hydration.
A dehydration reaction is aeration when any organic substance loses the water molecule to form an alkene.
Answer to Problem 14.29P
True.
Explanation of Solution
The oxidation of the primary alcohols gives either aldehyde or carboxylic acid, depending on the experimental condition. For illustration, the primary alcohol in an acid catalyzed oxidation gives an aldehyde and further acid catalyzed oxidation gives a corresponding acid.
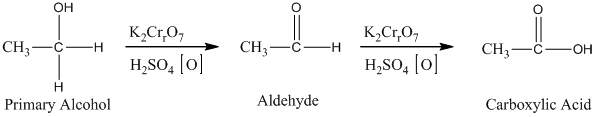
Therefore, this statement is True.
(h)
Interpretation:
State True or false.
The oxidation of a secondary alcohol gives a carboxylic acid.
Concept Introduction:
Chemical reaction is the procedure to transform the chemical substance in the new substance. During this procedure sometimes, acid is involved which is the substance which could either accept electron pairs or donate protons in the reactions.
A catalyst is the molecule which is used to speed up the chemical reaction, not being consumed within the procedure. Acids are used as catalysts are needed for an acid catalyzed hydration.
A dehydration reaction is aeration when any organic substance loses the water molecule to form an alkene.
Answer to Problem 14.29P
False.
Explanation of Solution
The secondary alcohol in an acid-catalyzed oxidation gives ketone. The oxidation of the secondary alcohol gives ketone in the presence of potassium chromate as an oxidizing agent. It does not produce carboxylic acid because it lacks a hydrogen atom.
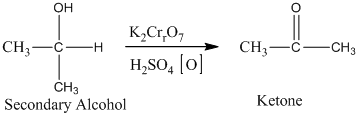
Therefore, this statement is false.
(i)
Interpretation:
State True or false.
Acetic acid, CH3 COOH, can be prepared from ethylene, CH2 =CH2, by treatment of ethylene with H2 O/H2 SO4, followed by treatment with K2 Cr2 O7 /H2 SO4.
Concept Introduction:
Chemical reaction is the procedure to transform the chemical substance in the new substance. During this procedure sometimes, acid is involved which is the substance which could either accept electron pairs or donate protons in the reactions.
A catalyst is the molecule which is used to speed up the chemical reaction, not being consumed within the procedure. Acids are used as catalysts are needed for an acid catalyzed hydration.
A dehydration reaction is aeration when any organic substance loses the water molecule to form an alkene.
Answer to Problem 14.29P
True.
Explanation of Solution
Ethylene undergoes acid catalyzed hydration to produce ethanol. This, ethanol, in further treatment with potassium dichromate, undergoes oxidation to form acetic acid. The reaction is.
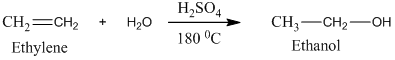
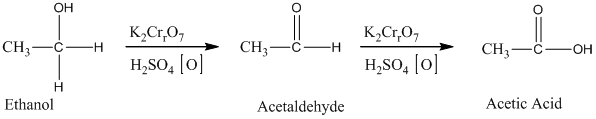
Therefore, this statement is True.
(j)
Interpretation:
State True or false.
Treatment of propene, CH3 CH=CH2, with H2 O/H2 SO4, followed by treatment with K2 Cr2 O7 /H2 SO4 gives propanoic acid.
Concept Introduction:
Chemical reaction is the procedure to transform the chemical substance in the new substance. During this procedure sometimes, acid is involved which is the substance which could either accept electron pairs or donate protons in the reactions.
A catalyst is the molecule which is used to speed up the chemical reaction, not being consumed within the procedure. Acids are used as catalysts are needed for an acid catalyzed hydration.
A dehydration reaction is aeration when any organic substance loses the water molecule to form an alkene.
Answer to Problem 14.29P
False.
Explanation of Solution
The hydration of propene with water in the presence of sulfuric acid produces propanol. It is a secondary alcohol. Propanol undergoes oxidation in the presence of potassium chromate and produces propanone. There is no hydrogen atom present in propanone. So, it does not produce propanoic acid in oxidation.
Therefore, this statement is false.
Want to see more full solutions like this?
Chapter 14 Solutions
Introduction to General, Organic and Biochemistry
- 14-49 Answer true or false. Today, the major carbon sources for the synthesis of methanol are coal and methane (natural gas), both nonrenewable resources. Today the major carbon sources for the synthesis of ethanol are petroleum and natural gas, both nonrenewable resources. Intermolecular acid-catalyzed dehydration of ethanol gives diethyl ether. Conversion of ethylene to ethylene glycol involves oxidation to ethylene oxide, followed by acid-catalyzed hydration (addition of water, to ethylene oxide. Ethylene glycol is soluble in water in all proportions. A major use of ethylene glycol is as automobile antifreeze.arrow_forward14-78 Consider alkenes A, B, and C. each of which has the same molecular formula, C(.H12. Alkenes B and C can each be separated into cis and trans isomers. Upon catalytic reduction using H,, in the presence of a transition metal catalyst (Ni, Pd, or Pt>, alkenes A, B, and C all give hexane as the only product. Acid- catalyzed hydration of alkene C gives one alcohol with the molecular formula CeH14O. Acid catalyzed- hydration of alkene B gives an equal mixture of two alcohols, each with the molecular formula C6H14O. Acid-catalyzed hydration of alkene C gives only a single alcohol with the molecular formula C6H14O. Propose structural formulas for alkenes A, B, and C and the alcohols formed by acid-catalyzed hydration of each, consistent with these experimental results.arrow_forward13-25 Answer true or false. (a) Phenols and alcohols have in common the presence of an —OH group. Phenols are weak acids and react with strong bases to give water-soluble salts. The pK„ of phenol is smaller than that of acetic acid. Autoxidation converts an R—H group to an R—OH group. A carbon radical has only seven electrons in the valence shell of one of its carbons, and this carbon bears a positive charge. (f, A characteristic of a chain initiation step is conversion of a nonradical to a radical. Autoxidation is a radical-chain reaction. A characteristic of the chain propagation step is reaction of a radical and a molecule to form a new radical and a new molecule. Vitamin E and other natural antioxidants function by interrupting the cycle of chain propagation steps that occurs in autoxidation.arrow_forward
- 14-71 The mechanism of the acid-catalyzed dehydration of an alcohol to an alkene is the reverse of the acid- catalyzed hydration of an alkene. The dehydration mechanism occurs by the following three steps. Step 1: Add a proton. Step 2: Break a bond to form stable molecules or ions. Step 3: Take away a proton. These three steps are illustrated here by the dehydration of 2-butanol to give 2-butene. Use curved arrows to show the flow of electrons in each step; that is, show how each bond-making or bond-breaking step occurs. H I + Step 3: CH3—CH—CH—CH3 CH3—CH=CH—CH3 + H + 2-butenearrow_forward17-73 Alcohols can be prepared by the acid-catalyzed hydration of alkenes (Section 12-6B) and by the reduction of aldehydes and ketones (Section 17-4B). Show how you might prepare each of the following alcohols by (1) acid-catalyzed hydration of an alkene and (2) reduction of an aldehyde or a ketone. (a) Ethanol (b) Cyclohexanol (c) 2-Propanol (d) 1-Phenylethanolarrow_forward13-23 What reagents and/or catalysts are necessary to carry out each conversion? Each conversion requires two steps. Benzene to 3-nitrobenzenesulfonic acid Benzene to l-bromo-4-chlorobenzenearrow_forward
- 14-9 What is the difference in structure between a primary, a secondary, and a tertiary alcohol?arrow_forward14-73 Lipoic acid is a growth factor for many bacteria and protozoa and an essential component of several enzymes involved in human metabolism. COOH Lipoic acid Name the two functional groups in lipoic acid. At one stage in its function in human metabolism, the disulfide bond of lipoic acid is reduced to two thiol groups. Draw a structural formula for this reduced form of lipoic acid.arrow_forward14-17 Explain in terms of noncovalent interactions why the low-molecular-weight alcohols are soluble in water but the low-molecular-weight alkanes and alkynes are not.arrow_forward
- 13-22 What reagents and/or catalysts are necessary to carry out each conversion? Benzene to nitrobenzene 1,4-dichlorobenzene to 2-bromo-l,4-dichlorobenzene Benzene to anilinearrow_forward14-34 Write equations for the reaction of 2-butanol with these reagents. H2SO4, heat K2Cr2O7, H2SO4arrow_forwardDraw the structural formula of an alkene that undergoes acid-catalyzed hydration to give each of the following alcohols as the major product. More than one alkene may give each compound as the major productarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
