
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 14.71P
14-71 The mechanism of the acid-catalyzed dehydration of an alcohol to an
Step 1: Add a proton.
Step 2: Break a bond to form stable molecules or ions. Step 3: Take away a proton.
These three steps are illustrated here by the dehydration of 2-butanol to give 2-butene. Use curved arrows to show the flow of electrons in each step; that is, show how each bond-making or bond-breaking step occurs.
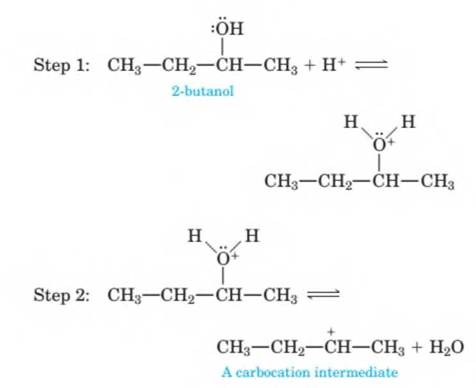
H
I +
Step 3: CH3—CH—CH—CH3
CH3—CH=CH—CH3 + H +
2-butene
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 14 Solutions
Introduction to General, Organic and Biochemistry
Ch. 14.1 - Prob. 14.1PCh. 14.1 - Prob. 14.2PCh. 14.2 - Problem 14-3 Draw structural formulas for the...Ch. 14.2 - Prob. 14.4PCh. 14.2 - Prob. 14.5PCh. 14.3 - Problem 14-6 Write the common name for each ether.Ch. 14.4 - Prob. 14.7PCh. 14 - 14-8 Answer true or false. The functional group of...Ch. 14 - 14-9 What is the difference in structure between a...Ch. 14 - 14-10 Which of the following are secondary...
Ch. 14 - 14-11 Which of the alcohols in Problem 14-10 are...Ch. 14 - 14-12 Write the 1UPAC name of each compound. (e)...Ch. 14 - Prob. 14.13PCh. 14 - Prob. 14.14PCh. 14 - 14-15 Both alcohols and phenols contain an —OH...Ch. 14 - Prob. 14.16PCh. 14 - 14-17 Explain in terms of noncovalent interactions...Ch. 14 - Prob. 14.18PCh. 14 - Prob. 14.19PCh. 14 - 14-20 Show hydrogen bonding between methanol and...Ch. 14 - 14-21 Show hydrogen bonding between the oxygen of...Ch. 14 - 14-22 Arrange these compounds in order of...Ch. 14 - 14-23 Arrange these compounds in order of...Ch. 14 - 14-24 2-Propanol (isopropyl alcohol) is commonly...Ch. 14 - 14-25 Explain why glycerol is much thicker (more...Ch. 14 - Prob. 14.26PCh. 14 - Prob. 14.27PCh. 14 - 14-28 Give the structural formula of an alkene or...Ch. 14 - Prob. 14.29PCh. 14 - 14-30 Show how to distinguish between cyclohexanol...Ch. 14 - 14-31 Compare the acidity of alcohols and phenols,...Ch. 14 - 14-32 Both 2,6-diisopropylcyclohexanol and the...Ch. 14 - 14-33 Write equations for the reaction of...Ch. 14 - 14-34 Write equations for the reaction of...Ch. 14 - 14-35 Write equations for the reaction of each of...Ch. 14 - 14-36 Show how to convert cyclohexanol to these...Ch. 14 - Prob. 14.37PCh. 14 - Prob. 14.38PCh. 14 - 14-39 Name two important alcohols derived from...Ch. 14 - 14-40 Name two important alcohols derived from...Ch. 14 - Prob. 14.41PCh. 14 - 14-42 Write the common name for each ether. ch3...Ch. 14 - Prob. 14.43PCh. 14 - 14-44 Answer true or false. (a) The functional...Ch. 14 - Prob. 14.45PCh. 14 - Prob. 14.46PCh. 14 - 14-47 Following are structural formulas for...Ch. 14 - 14-48 Explain why methanethiol, CH3SH, has a lower...Ch. 14 - 14-49 Answer true or false. Today, the major...Ch. 14 - Prob. 14.50PCh. 14 - 14-51 (Chemical Connections 14B) When was...Ch. 14 - 14-52 (Chemical Connections 14B) What was Alfred...Ch. 14 - Prob. 14.53PCh. 14 - 14-54 (Chemical Connections 14C) What is the color...Ch. 14 - 14-55 (Chemical Connections 140 The legal...Ch. 14 - 14-56 (Chemical Connections 14D) What does it mean...Ch. 14 - 14-57 (Chemical Connections 14E) What are the...Ch. 14 - Prob. 14.58PCh. 14 - Prob. 14.59PCh. 14 - 14-60 Write a balanced equation for the complete...Ch. 14 - 14-61 Knowing what you do about electronegativity,...Ch. 14 - 14-62 Draw structural formulas and write IUPAC...Ch. 14 - Prob. 14.63PCh. 14 - 14-64 Explain why the boiling point of ethylene...Ch. 14 - Prob. 14.65PCh. 14 - 14-66 1,4-Butanediol, hexane, and 1-pentanol have...Ch. 14 - 14-67 Of the three compounds given in Problem...Ch. 14 - Prob. 14.68PCh. 14 - 14-69 Show how to prepare each compound from...Ch. 14 - 14-70 Show how to prepare each compound from...Ch. 14 - 14-71 The mechanism of the acid-catalyzed...Ch. 14 - Prob. 14.72PCh. 14 - 14-73 Lipoic acid is a growth factor for many...Ch. 14 - 14-74 Following is a structural formula for the...Ch. 14 - Prob. 14.75PCh. 14 - Prob. 14.76PCh. 14 - Prob. 14.77PCh. 14 - 14-78 Consider alkenes A, B, and C. each of which...Ch. 14 - Prob. 14.79P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 14-34 Write equations for the reaction of 2-butanol with these reagents. H2SO4, heat K2Cr2O7, H2SO4arrow_forward14-36 Show how to convert cyclohexanol to these compounds. Cyclohexene Cyclohexane Cyclohexanone Bromocyclohexanearrow_forward14-30 Show how to distinguish between cyclohexanol and cyclohexene by a simple chemical test. Tell what you would do, what you would expect to see, and how you would interpret your observation.arrow_forward
- 14-33 Write equations for the reaction of 1-butanol, a primary alcohol, with these reagents. H2SO4, heat K2Cr2O7, H2SO4arrow_forward14-69 Show how to prepare each compound from 2-methyl-1 -propanol. 2-Methylpropene 2-Methyl-2-propanol 2-Methylpropanoic acid, (CH3)2CHCOOHarrow_forward14-20 Show hydrogen bonding between methanol and water in the following ways. Between the oxygen of methanol and a hydrogen of water Between the hydrogen of methanol’s OH group and the oxygen of waterarrow_forward
- 14-70 Show how to prepare each compound from 2-methylcyclohexanol.arrow_forward13-25 Answer true or false. (a) Phenols and alcohols have in common the presence of an —OH group. Phenols are weak acids and react with strong bases to give water-soluble salts. The pK„ of phenol is smaller than that of acetic acid. Autoxidation converts an R—H group to an R—OH group. A carbon radical has only seven electrons in the valence shell of one of its carbons, and this carbon bears a positive charge. (f, A characteristic of a chain initiation step is conversion of a nonradical to a radical. Autoxidation is a radical-chain reaction. A characteristic of the chain propagation step is reaction of a radical and a molecule to form a new radical and a new molecule. Vitamin E and other natural antioxidants function by interrupting the cycle of chain propagation steps that occurs in autoxidation.arrow_forward14-15 Both alcohols and phenols contain an —OH group. What structural feature distinguishes these two classes of compounds? Illustrate your answer by drawing the structural formulas of a phenol with six carbon atoms and an alcohol with six carbon atoms.arrow_forward
- 14-78 Consider alkenes A, B, and C. each of which has the same molecular formula, C(.H12. Alkenes B and C can each be separated into cis and trans isomers. Upon catalytic reduction using H,, in the presence of a transition metal catalyst (Ni, Pd, or Pt>, alkenes A, B, and C all give hexane as the only product. Acid- catalyzed hydration of alkene C gives one alcohol with the molecular formula CeH14O. Acid catalyzed- hydration of alkene B gives an equal mixture of two alcohols, each with the molecular formula C6H14O. Acid-catalyzed hydration of alkene C gives only a single alcohol with the molecular formula C6H14O. Propose structural formulas for alkenes A, B, and C and the alcohols formed by acid-catalyzed hydration of each, consistent with these experimental results.arrow_forward14-48 Explain why methanethiol, CH3SH, has a lower boiling point (6°C) than methanol, CH3OH (65°C), even though methanethiol has a higher molecular weightarrow_forward14-35 Write equations for the reaction of each of the fol lowing compounds with K2Cr2O7/H2SO4. 1-Octanol 1,4-Butanediolarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY