
(a)
Interpretation:
The structural formula of methanal is to be drawn.
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent
Answer to Problem 14.7E
The structural formula of methanal is,
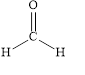
Explanation of Solution
The given name is methanal. The functional group present in the given compound is aldehyde

Figure 1
The structural formula of methanal is shown in Figure 1.
(b)
Interpretation:
The structural formula of
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
Aldehydes and ketones contain carbonyl ![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
Answer to Problem 14.7E
The structural formula of
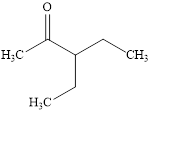
Explanation of Solution
The given name is

Figure 2
The structural formula of
(c)
Interpretation:
The structural formula of
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
Aldehydes and ketones contain carbonyl ![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
Answer to Problem 14.7E
The structural formula of
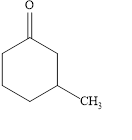
Explanation of Solution
The given name is

Figure 3
The structural formula of
(d)
Interpretation:
The structural formula of
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
Aldehydes and ketones contain carbonyl ![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
Answer to Problem 14.7E
The structural formula of
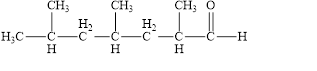
Explanation of Solution
The given name is

Figure 4
The structural formula of
Want to see more full solutions like this?
Chapter 14 Solutions
CHEMISTRY FOR TODAY+OWLV2 24 MO>IP<
- List the following compounds in order of increasing water solubility: a.ethoxyethane b.propanoic acid c.pentane d.1 butanolarrow_forwardTollen's test is a qualitative laboratory test used to distinguish between aldehydes and ketones. The reagent uses an aqueous solution of _____________. Aliphatic compounds would produce what color in Brady's test Jones Oxidation test uses ________ and sulfuric acid as reagentsarrow_forwardHow would you convert 3-ethyl-2-pentene to 3-ethyl-3-pentanol. List the reagentsarrow_forward
- Grignard reagents can react with ester functional groups to form tertiary alcohols. What is the final product of the following reaction?arrow_forwardPlease draw out structural formula below: 1. 4-Methyl-3-penten-2-one 2. 4-Hydroxy-4-methyl-2-pentanone 3. 2,5-Dimethylcyclohexanonearrow_forwardConsider a compound with a formula: C5H10O (5 carbons, 10 hydrogens, one oxygen). A correct IUPAC name for one of ketone isomers of this compound is a 3-Methybutanal b 4-Methylbutanone c 3-Methylbutanone d None of the abovearrow_forward
- When & how Biological oxidation of an alcohol occurs ?arrow_forward"A research team synthesizes a novel organic compound 'X' with the molecular formula C5H8O2. When 'X' is treated with a deuterated acid (D2O), a single deuterium atom replaces a hydrogen atom, forming compound 'Y' (C5H7DO2). 'X' does not react with 2,4-Dinitrophenylhydrazine (2,4-DNP) but does react with both Tollens' reagent and Benedict's solution, forming a silver mirror and a red precipitate, respectively. Furthermore, 'X' undergoes catalytic hydrogenation over a palladium catalyst, consuming one mole of hydrogen to form a compound 'Z' (C5H10O2). Based on these observations, what is the most likely structure of compound 'X'?" A. Methyl vinyl ketone B. 3-Buten-2-one C. Acetoacetic ester D. 2-Hydroxypent-3-enalarrow_forwardWrite structural formulas for the following compounds (includes both old- and new-style names).(a) 2-octyne (b) ethylisopentylacetylene (c) ethynylbenzene(d) cyclohexylacetylene (e) 5-methyl-3-octyne (f) trans-3,5-dibromocyclodecynearrow_forward
- Fill in the blanks; ______ has a higher boiling point than _________? a. Carbon dioxide; 1-butanamine b. Butane; 2-butanol c. None of these are correct d. 1-methoxypropane; 2-heptene e. Cyclohexane; 1-propanolarrow_forwardWhat kind of solvent ingredients is usually used in the concentrations of 4-10 percent in skin care products and their function is to soften skin cells and to lessen wrinkles? A. Ethly acetate B. Alpha hydroxyl acids C. Phenols and phenol derivatives D. Aliphatic alcoholsarrow_forwardDraw structural formulas for the following: a. methyl isopropyl ether b. phenyl propyl ether c. 2-methoxypentane d. 1,2-diethoxycyclopentane e. 2-phenoxy-2-butenearrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





