
(a)
Interpretation:
The structural formula of propanal is to be drawn.
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent
Answer to Problem 14.8E
The structural formula of propanal is,
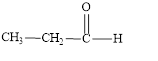
Explanation of Solution
The given name is propanal. The functional group present in the given compound is aldehyde
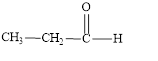
Figure 1
The structural formula of propanal is shown in Figure 1.
(b)
Interpretation:
The structural formula of
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
Aldehydes and ketones contain carbonyl ![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
Answer to Problem 14.8E
The structural formula of
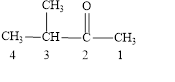
Explanation of Solution
The given name is
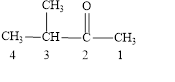
Figure 2
The structural formula of
(c)
Interpretation:
The structural formula of
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
Aldehydes and ketones contain carbonyl ![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
Answer to Problem 14.8E
The structural formula of
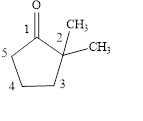
Explanation of Solution
The given name is

Figure 3
The structural formula of
(d)
Interpretation:
The structural formula of
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
• First identify the word root for the given compound.
• The suffix used in the compound like –ane, ene, yne, ol, al and so on.
• Identify the position, location, and number of the substituent bonded to the carbon chain.
Aldehydes and ketones contain carbonyl ![]() functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
functional group in their parent chain and are named by adding suffix –al and –one to the name of the parent alkane.
Answer to Problem 14.8E
The structural formula of
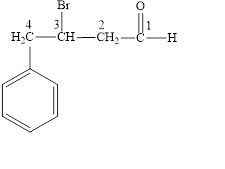
Explanation of Solution
The given name is

Figure 4
The structural formula of
Want to see more full solutions like this?
Chapter 14 Solutions
LMS Integrated OWLv2, 4 terms (24 months) Printed Access Card for Seager/Slabaugh/Hansen’s Chemistry for Today: General, Organic, and Biochemistry, 9th
- Draw the structures of the chief product formed when the following alcohols are dehydrated to alkenes: a. b.arrow_forwardHydroCarbon Organic Reactions: 1. Write an equation for the preparation of each of the following compounds a) 2,2-dichloropentane b) 1,2-dichloropentane c) 2,3,3-trichlorobutanearrow_forwardWhich of the following has highest boiling point? a) 2-hexanone b) 2-pentanone c) butanoic acid d) 3-methyl-2-butanonearrow_forward
- Draw a structure for each of the following: a. m-chloromethylbenzene c. o-nitroaniline e. m-dichlorobenzene b. p-bromophenol d. m-chlorobenzonitrile f. o-xylenearrow_forwardWhy must a dilute solution of phenol be used for disinfecting environmental surfaces?arrow_forwardWhat structural characteristic is shared by the aldehydes and the ketones? A) They both are straight chain compounds. B) Aldehydes and ketones both contain a carbonyl carbon. C) Both of these compound classes have as the smallest compound a 5 carbon skeleton. D) Aldehydes and ketones have no shared characteristics.arrow_forward
- Select the functional group with the lowest oxidation state. a. carboxylic acid b. nitrile c. alkene d. ketonearrow_forwardDraw the product resulting from mild oxidation of (a) 2-butanol; (b) 2-methylpropanal; (c) cyclopentanol.arrow_forwardFill in the blanks; ______ has a higher boiling point than _________? a. Carbon dioxide; 1-butanamine b. Butane; 2-butanol c. None of these are correct d. 1-methoxypropane; 2-heptene e. Cyclohexane; 1-propanolarrow_forward
- Draw the structures of the following compounds:(a) Ethanoic acid(b) Bromopentane(c) Butanonearrow_forwardWrite the structural formula for each of the following compounds a) 1-phenyl-2-butanone b) cyclopentanonearrow_forwardDescribes the disposal procedure for the following substances : A- Formaldehyde B- Propofol C- Pentobarbitalarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





