
Biochemistry: Concepts and Connections (2nd Edition)
2nd Edition
ISBN: 9780134763040
Author: APPLING
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 3P
Freshly prepared mitochondria were incubated with ß-hydroxybutyrate, oxidized cytochrome c, ADP, Pi, and cyanide. ß-hydroxybutyrate is oxidized by an NAD+ -dependent dehydrogenase. 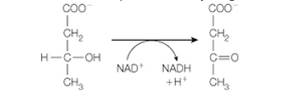
- The experimenter measured the rate of oxidation of ß-hydroxybutyrate and the rate of formation of ATP.
- Indicate the probable flow of electrons in this system.
- How many moles of ATP would you expect to be formed per mole of ß-hydroxybutyrate oxidized in this system?
- Why is ß-hydroxybutyrate added rather than NADH?
- What is the function of the cyanide?
- Write a balanced equation for the overall reaction occurring in this system (electron transport and ATP synthesis).
- Calculate the net standard free energy change (ΔGO') in this system, using
E'o values from Table 14.1 and a ΔGO' value for ATP hydrolysis of -32.2 kJ / mol.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Freshly prepared mitochondria were incubated with B-hyroxybutyrate,
oxidized cytochrome c, ADP, P, and cyanide. B-Hyroxybutyrate is oxidized
by an NAD*-dependent dehydrogenase.
CoO
COO
CH,
CH,
H-C-OH
C=0
NAD+
NADH
CH,
+H+
CH,
The experimenter measured the rate of oxidation of B-hyroxybutyrate and
the rate of formation of ATP.
(a) Indicate the probable flow of electrons in this system.
(b) How many moles of ATP would you expect to be formed per mole of
B-hyroxybutyrate oxidized in this system?
(c) Why is B-hyroxybutyrate added rather than NADH?
(d) What is the function of the cyanide?
(e) Write a balanced equation for the overall reaction occurring in this
system (electron transport and ATP synthesis).
(f) Calculate the net standard free energy change (AG") in this system,
using E', values from Table 14.1 and a AG" value for ATP hydrolysis of
-32.2 kJ/mol.
When pure reduced cytochrome c is added to carefully prepared
mitochondria along with ADP, P, antimycin A, and oxygen, the
cytochrome c becomes oxidized, and ATP is formed, with a P/o
ratio approaching 1.0.
(a) Indicate the probable flow of electrons in this system.
(b) Why was antimycin A added?
(c) What does this experiment tell you about the location of cou-
pling sites for oxidative phosphorylation?
(d) Write a balanced equation for the overall reaction (including cyt
c oxidation and ATP synthesis).
A newly identified bacterium is unable to synthesize ubiquinone. A mobile electron carrier called CXC3 is used as a substitute. From the information provided in the table, calculate the delta G knot prime and the Keq value at 298 K for the redox reaction that occurs in this bacterium’s electron transport chain.
Explain the impact that using CXC 3 instead of ubiquinol will have on ATP production in the cell. How might this cell adapt to this situation?
Chapter 14 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Ch. 14 - Prob. 1PCh. 14 - When pure reduced cytochrome c is added to...Ch. 14 - Freshly prepared mitochondria were incubated with...Ch. 14 - Prob. 4PCh. 14 - Prob. 5PCh. 14 - Prob. 6PCh. 14 - Intramitochondrial ATP concentrations are about 5...Ch. 14 - Prob. 8PCh. 14 - Prob. 9PCh. 14 - Years ago there was interest in using uncouplers...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider a 24:1 △cis-9 fatty acid in the mitochondrion. For each fatty acid given, determine the following. 1. Gross ATP from b-oxidation cycles 2. Gross ATP from acetyl CoA produced 3. Gross ATP from conversion of propionyl CoA (if applicable) 4. Total number of ATP deducted 5. Total net ATParrow_forwardCalculate the ATP yield when glucose is catabolized completely to six CO2 by a eukaryotic microbe. How does this value compare to the ATP yield observed for a bacterium? Suppose a bacterium used the Entner-Doudoroff pathway to degrade glucose to pyruvate and then completed the catabolism of glucose via the TCA cycle. How would this affect the total maximum ATP yield? Explain your reasoning.arrow_forwardUnder standard conditions, is the oxidation of ubiquinol (Coenzyme Q) by O2 sufficiently exergonic to drive the synthesis of ATP? If yes, how many ATP can be synthesized assuming 100% efficiency?arrow_forward
- DCCD (diocyclohexylcarbodiimide) inhibits oxidative phosphorylation when the substrate is mitochondrial NADH. DCCD is a drug that binds to ATP synthase and blocks proton transport through the ion channel. a) Explain what the consequences of DCCD on cellular energy production are. b) Suggest at least one other cellular effect of DCCD and explain this effect.arrow_forwardComplete the sentence describing the pentose phosphate pathway in cells that require much more ribose 5-phosphate than NADPH. These cells need ribose 5-phosphate but have relatively higher concentrations of NADPH and lower concentrations of NADP*. Choose from the listed words to fill in the blanks: xylulose 5-phosphate, fructose 6-phosphate, glucose 6-phosphate, five, two, three, glyceraldehyde 3- phosphate, erythrose 4-phosphate, sedoheptulose 7-phosphate. One molecule of and two molecules of are used to generate molecules of ribose 5-phosphate by the reverse reactions of the nonoxidative phase of the pentose phosphate pathway.arrow_forwardIn the krebs cycle, if the enzyme used succinate dehydrogenase used NAD+ as the oxidizing agent, what impact would it have on the overall production of ATP in the call?arrow_forward
- Describe the chemiosmotic coupling mechanism. Draw a simple picture illustrating this mechanism and how ATP is synthesized in mitochondria. Label the components.arrow_forwardConsider 3 molecules of galactose: (write only the whole number; no decimal places) How many turns of Krebs Cycle will these molecules undergo for complete oxidation? b. How many moles of ATP are produced upon complete oxidation via malate-aspartate shuttle? c. If all the galactose molecules oxidize via pentose phosphate pathway (oxidative stage only), how many moles of NADPH will be produced?arrow_forwardIn order to metabolize lactose, most infants express the enzyme lactase in their intestines. How many pyruvate molecules and NADH molecules can be generated in glycolysis from one lactose molecule? Please explain. How many ATP molecules will be used and generated by 1 lactose molecule? Please explain.arrow_forward
- A new ATP-producing protein is discovered that couples ATP production to the oxidation of NADPH by oxidative phosphorylation. Assume that the value of ΔGo for ATP synthesis is 30 kJ•mol−1. If this protein only produces 1 molecule of ATP per reaction that consumes one NADPH: a. How much free energy is wasted, under standard conditions?b. How many more ATP molecules could be created by a perfectly efficient electron transport chain from one NADPH?arrow_forwardRefer to Table to explain why FAD rather than NAD+ is used in the succinate dehydrogenase reaction.arrow_forwardAssume that 2.5 ATPs are generated per NADH and 1.5 ATPs per FADH2. How many ATPs are generated from the FADH2 and NADH molecules from each repetition of the ββbeta-oxidation pathway? Express your answer as an integer.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
Mitochondrial mutations; Author: Useful Genetics;https://www.youtube.com/watch?v=GvgXe-3RJeU;License: CC-BY